Abstract
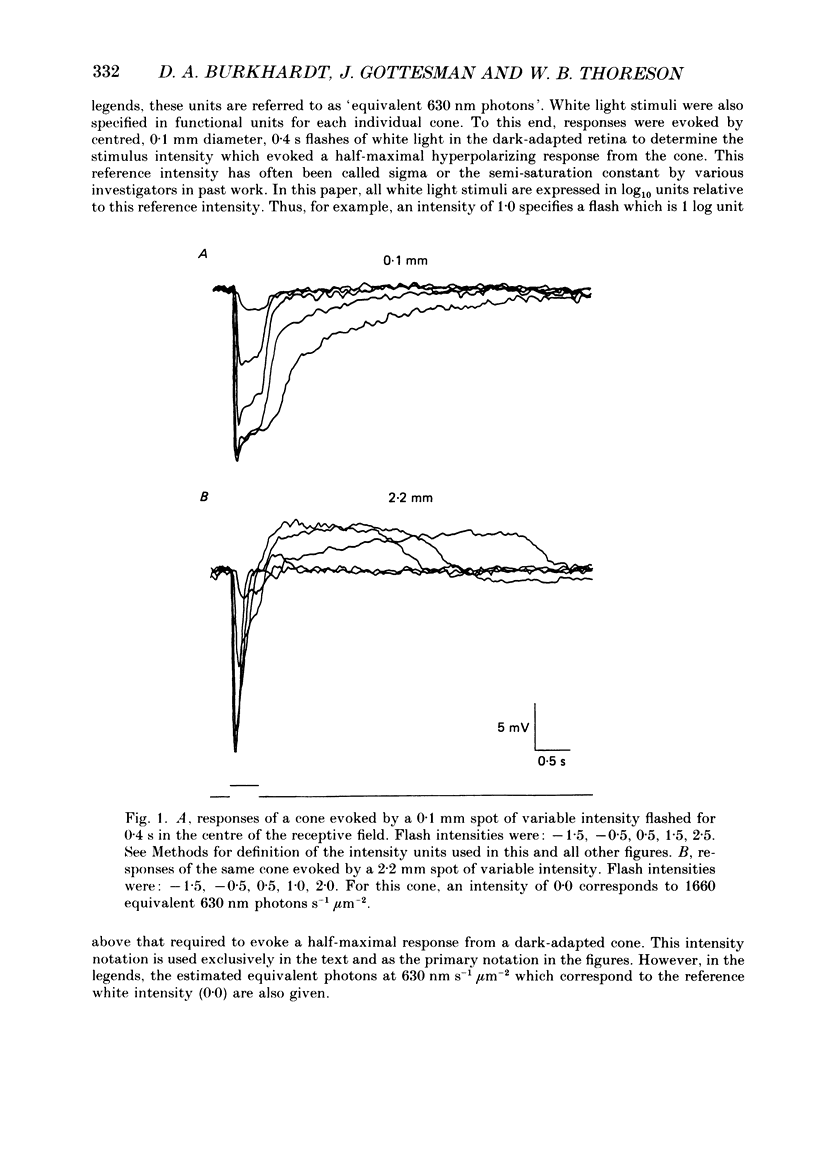
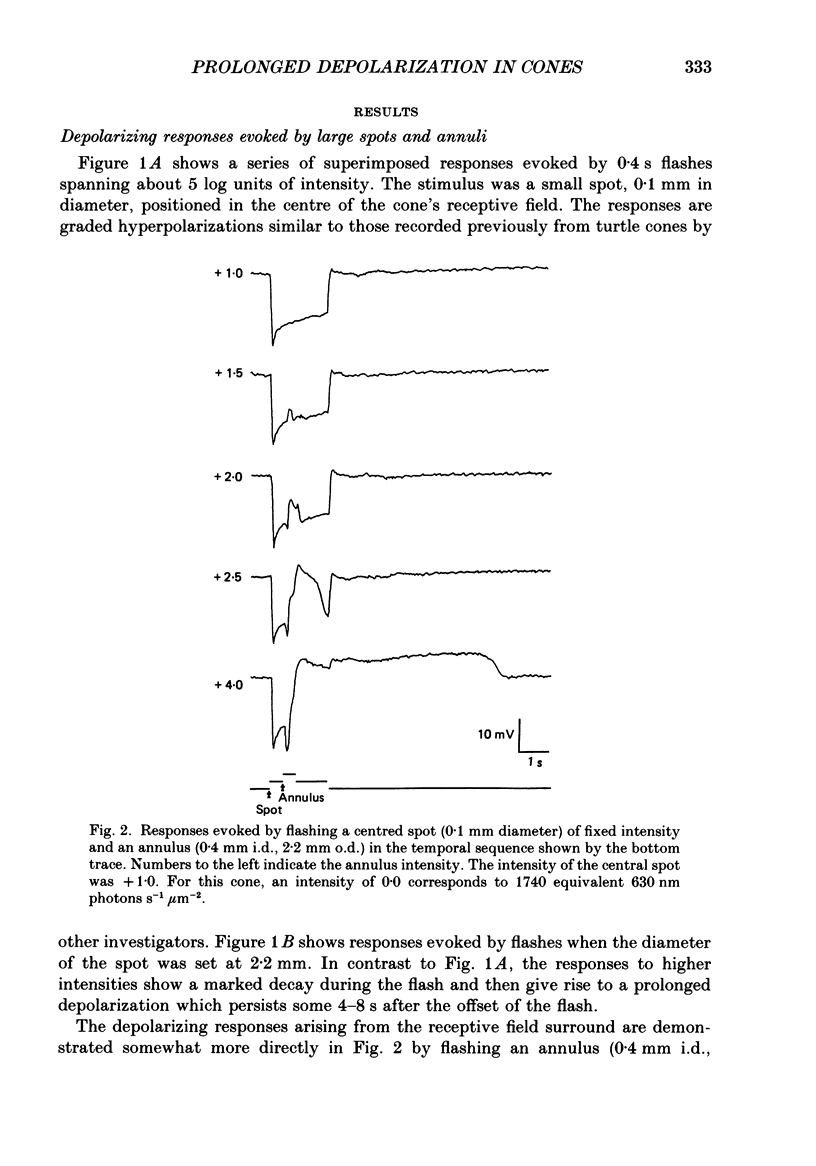
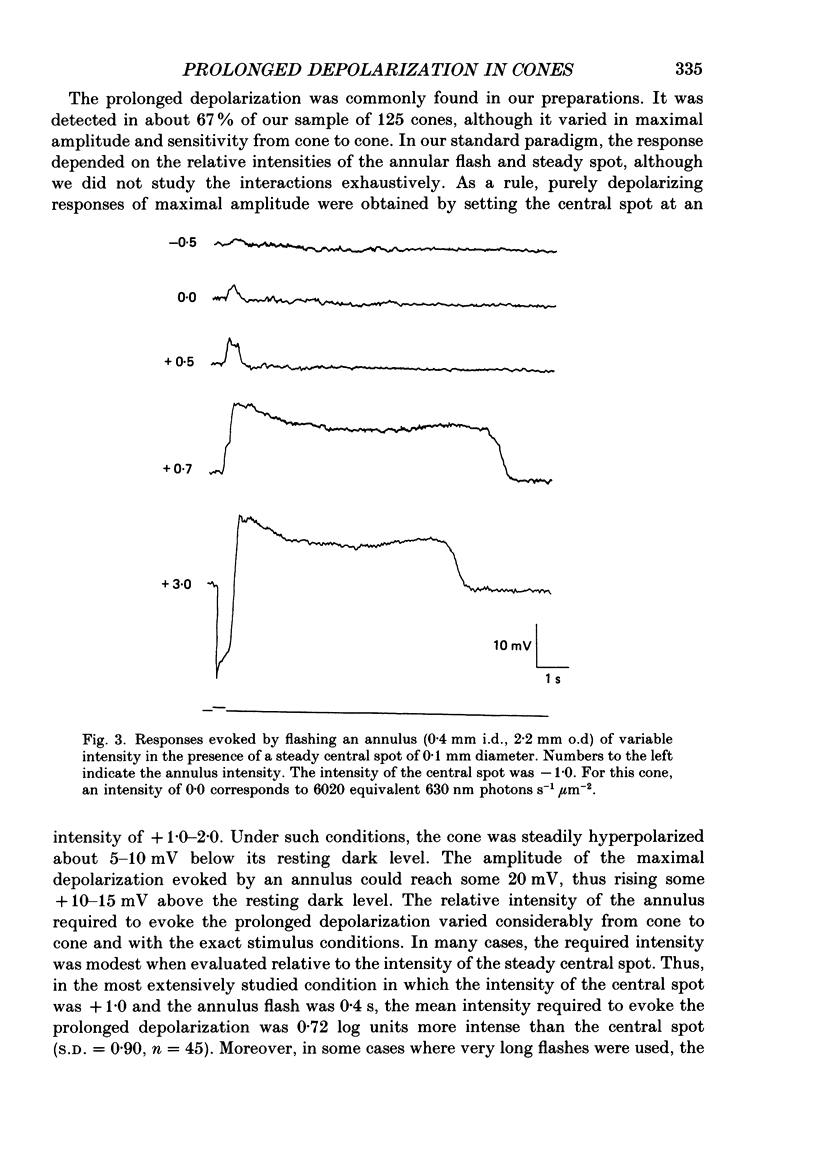
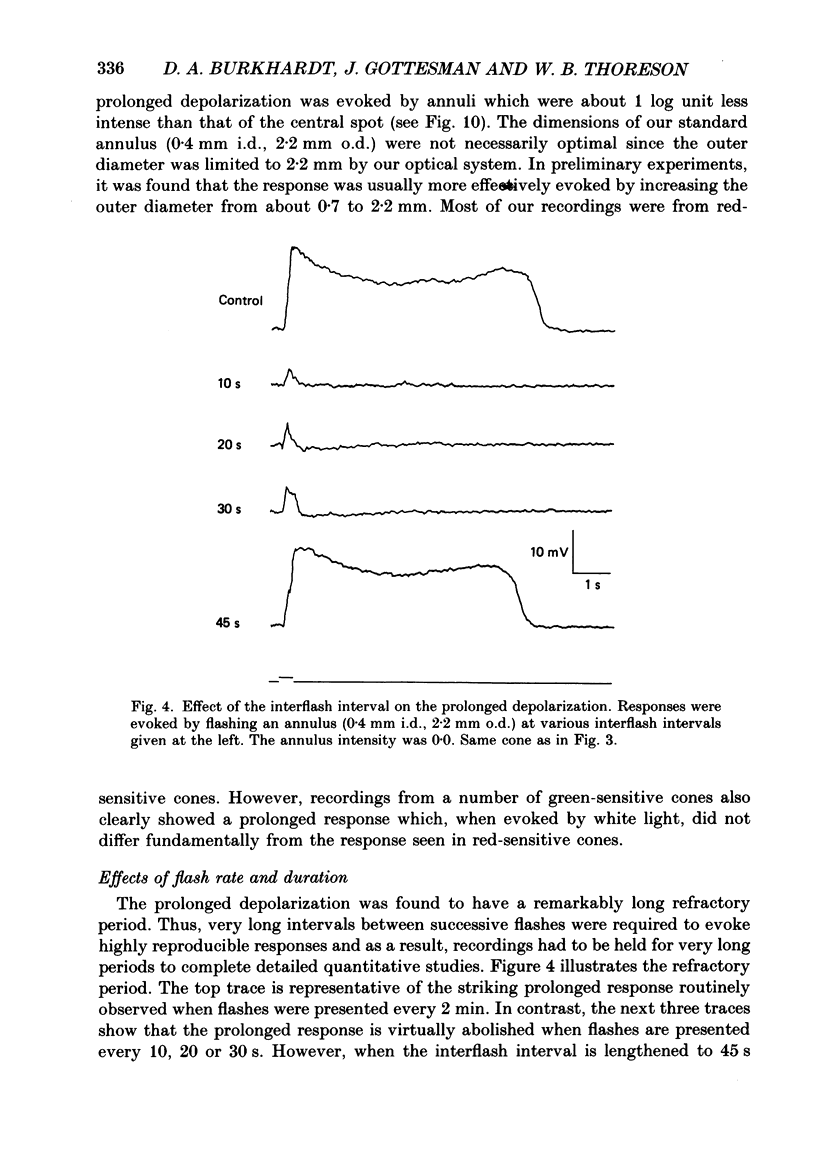
1. Responses evoked by stimulation of the receptive field surround were recorded intracellularly from cone photoreceptors in the retina of the turtle (Pseudemys scripta elegans). 2. A distinctive depolarizing response was evoked by flashing an annulus of light while steadily illuminating the centre of the receptive field. The response, here called 'the prolonged depolarization', was found in 67% of a sample of 125 cones and could reach some 20 mV in amplitude. 3. The prolonged depolarization is characterized by a set of properties which include: the capacity to persist up to 17 s after the flash, a stereotypical waveform, a long period of temporal facilitation, a very narrow dynamic range, and a long refractory period (30-45 s). 4. Depolarizing current pulses (0.01-0.1 nA) evoke a prolonged depolarization which is similar to and functionally interchangeable with that evoked by light. The prolonged depolarization is thus apparently generated by a voltage-sensitive mechanism intrinsic to the cone. 5. Brief depolarizing spikes were recorded in a small fraction of cones. The spikes appear to be dissociable from the prolonged depolarization although both might arise for similar regenerative mechanisms. 6. The prolonged depolarization is typically preceded by a graded, stimulus-locked depolarization which can also be recorded in isolation by flashing annuli of low intensity. The graded depolarization is probably a manifestation of the depolarizing influence arising from synaptic feed-back from horizontal cells first described by Baylor, Fuortes & O'Bryan (1971). 7. It is suggested that the graded depolarization triggers the prolonged depolarization and that complex responses arise from the interaction of these disparate components.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A., Gottesman J. Light adaptation and responses to contrast flashes in cones of the walleye retina. Vision Res. 1987;27(9):1409–1420. doi: 10.1016/0042-6989(87)90151-9. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A., Hassin G., Levine J. S., MacNichol E. F., Jr Electrical responses and photopigments of twin cones in the retina of the walleye. J Physiol. 1980 Dec;309:215–228. doi: 10.1113/jphysiol.1980.sp013505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Sensitization and centre-surround antagonism in Necturus retina. J Physiol. 1974 Feb;236(3):593–610. doi: 10.1113/jphysiol.1974.sp010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov A. L., Shura-Bura T. M. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 1986;26(1):33–44. doi: 10.1016/0042-6989(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Processing of visual signals in vertebrate photoreceptors. Arch Ital Biol. 1982 May;120(1-3):242–270. [PubMed] [Google Scholar]
- Daly S. J., Normann R. A. Temporal information processing in cones: effects of light adaptation on temporal summation and modulation. Vision Res. 1985;25(9):1197–1206. doi: 10.1016/0042-6989(85)90034-3. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Piccolino M. Sustained feedback effects of L-horizontal cells on turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):465–480. doi: 10.1098/rspb.1980.0008. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Hattori K., Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J Membr Biol. 1984;78(1):19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986 Apr;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft T. W., Burkhardt D. A. Telodendrites of cone photoreceptors: structure and probable function. J Comp Neurol. 1986 Jul 1;249(1):13–27. doi: 10.1002/cne.902490103. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Synaptic action mediating cone responses to annular illumination in the retina of the larval tiger salamander. J Physiol. 1981 Jan;310:205–214. doi: 10.1113/jphysiol.1981.sp013544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. A white-noise analysis of responses and receptive fields of catfish cones. J Neurophysiol. 1982 Jun;47(6):1057–1068. doi: 10.1152/jn.1982.47.6.1057. [DOI] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y., Nakatani K., Miyachi E., Watanabe S. GABA-mediated negative feedback from horizontal cells to cones in carp retina. Jpn J Physiol. 1982;32(6):911–926. doi: 10.2170/jjphysiol.32.911. [DOI] [PubMed] [Google Scholar]
- Murakami M., Takahashi K. Calcium action potential and its use for measurement of reversal potentials of horizontal cell responses in carp retina. J Physiol. 1987 May;386:165–180. doi: 10.1113/jphysiol.1987.sp016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman I., Normann R. A., Itzhaki A., Daly S. J. Chromatic and spatial information processing by red cones and L-type horizontal cells in the turtle retina. Vision Res. 1985;25(4):543–549. doi: 10.1016/0042-6989(85)90158-0. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):439–463. doi: 10.1098/rspb.1980.0007. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Lateral interactions in the outer plexiform layer of turtle retinas after atropine block of horizontal cells. Nature. 1977 Jul 21;268(5617):259–261. doi: 10.1038/268259a0. [DOI] [PubMed] [Google Scholar]
- Skrzypek J., Werblin F. Lateral interactions in absence of feedback to cones. J Neurophysiol. 1983 Apr;49(4):1007–1016. doi: 10.1152/jn.1983.49.4.1007. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981 Dec;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]


