Abstract
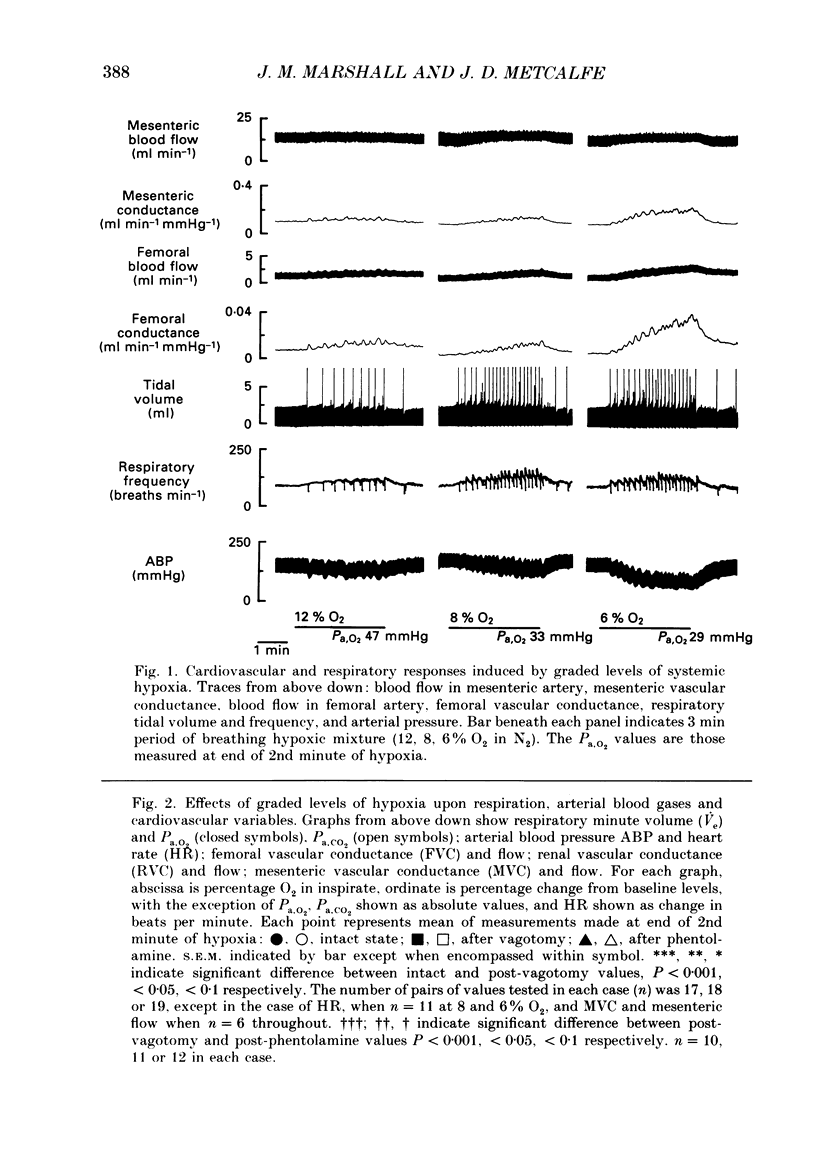
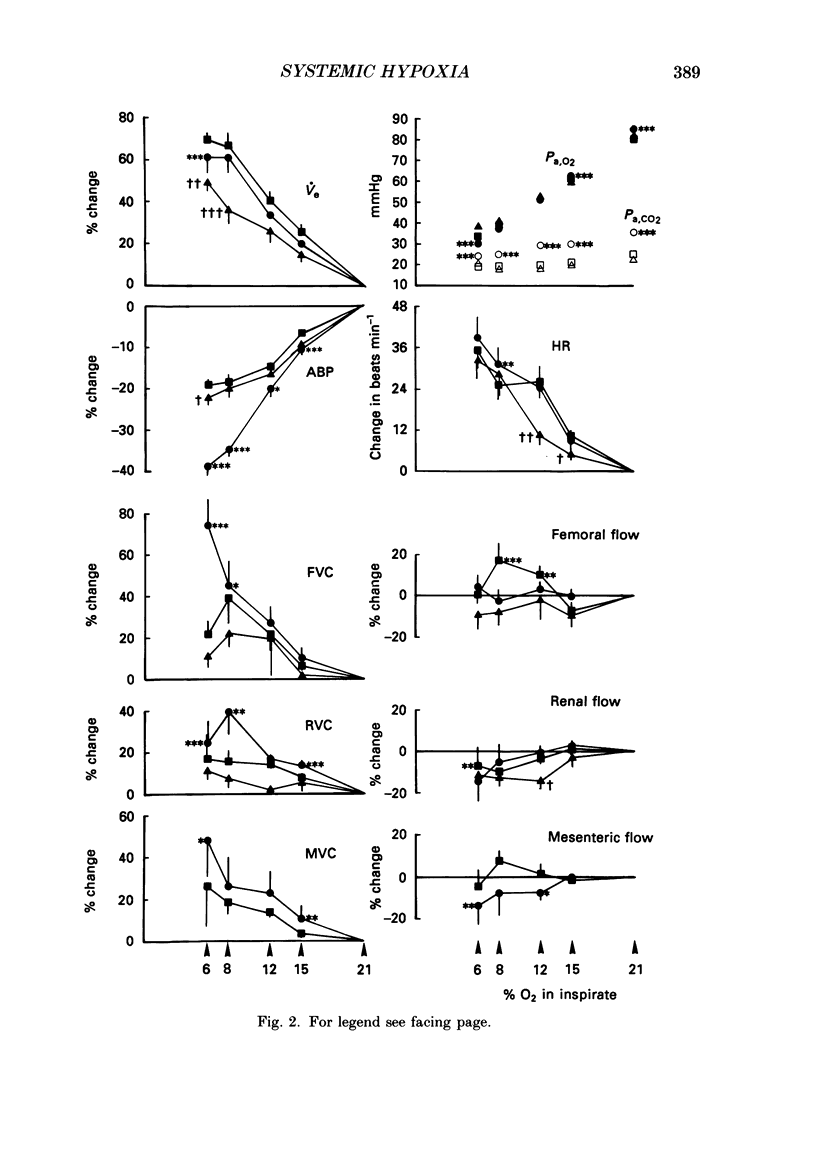
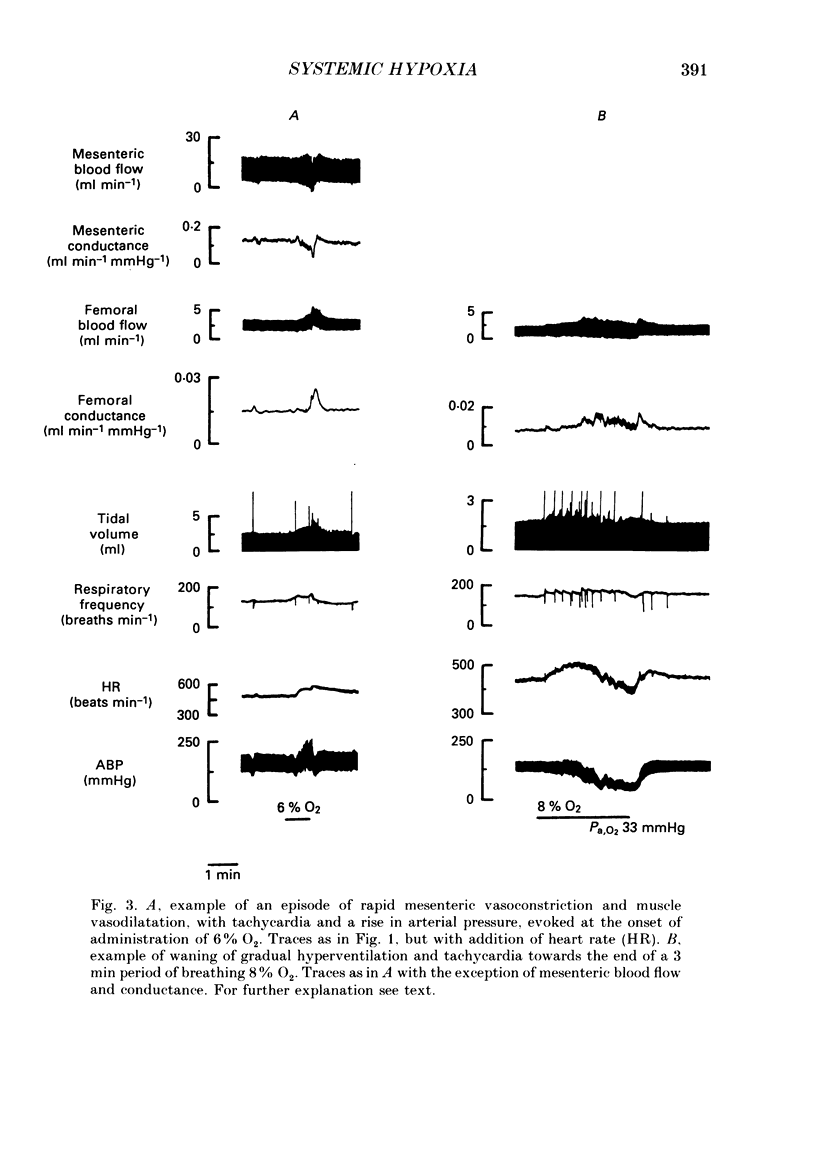
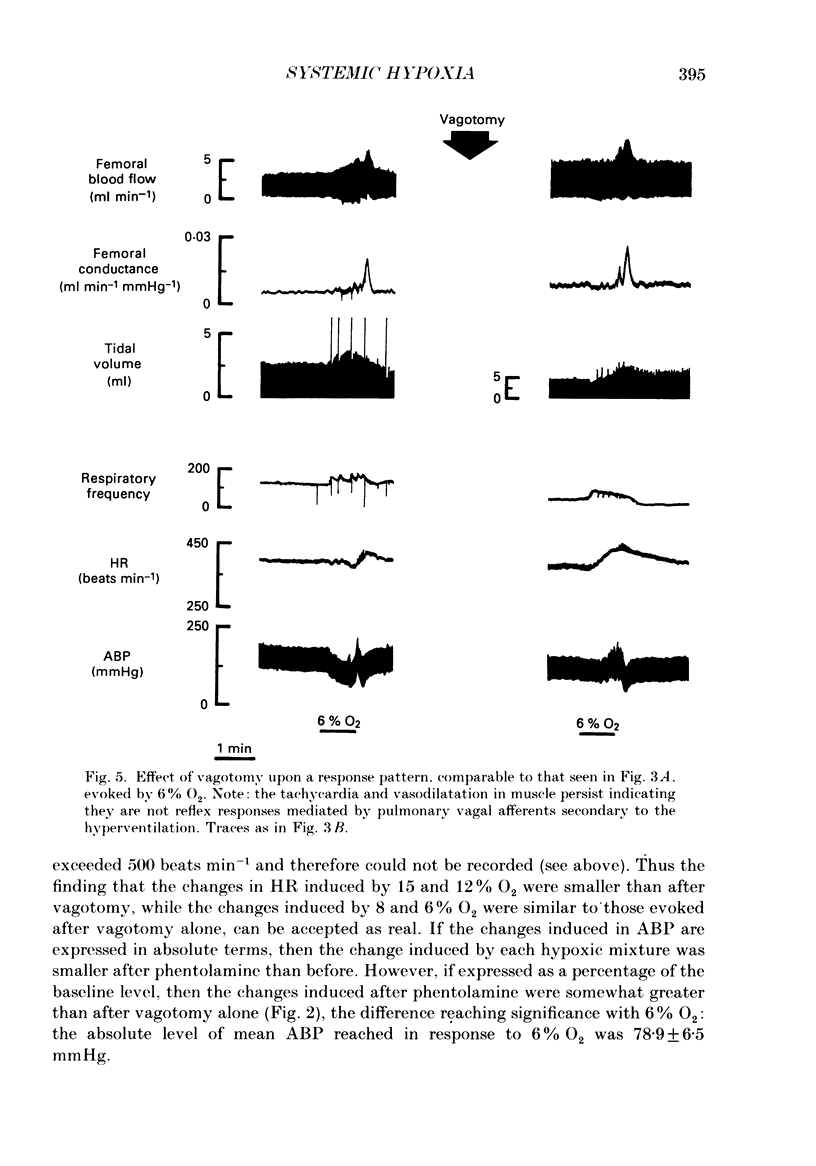
1. In rats anaesthetized with Saffan, we have further analysed the respiratory, cardiac and regional vascular responses induced by 3 min periods of graded hypoxia (breathing 15, 12, 8 or 6% O2 in N2). 2. Frequently, hypoxia evoked an episode, lasting 1-1.5 min, of tachycardia, renal and mesenteric vasoconstriction and skeletal muscle vasodilatation. The tachycardia and muscle vasodilatation persisted after vagotomy indicating they were not initiated by pulmonary stretch receptors secondary to hyperventilation. We propose that such episodes represented the cardiovascular components of the alerting-defence response initiated by activation of the brain stem defence areas by peripheral chemoreceptors. 3. Each of these episodes was superimposed upon gradual hyperventilation, tachycardia, fall in arterial pressure and vasodilatation in renal, mesenteric and muscle circulation the magnitudes of which at 2 min were generally graded with the level of hypoxia. In the 3rd minute, respiration and heart rate tended to wane below control levels. 4. Vagotomy had little effect on the heart rate changes and only slightly reduced the peripheral vasodilatation allowing the conclusion that the gradual tachycardia and peripheral vasodilatation was not a reflex initiated by pulmonary stretch receptors. 5. Guanethidine given after vagotomy abolished the tachycardia indicating it was sympathetically mediated; possible initiating factors are discussed. But the secondary bradycardia persisted indicating it reflected the direct effect of hypoxia on cardiac pacemaker tissue. 6. The peripheral vasodilatation persisted after guanethidine or phentolamine indicating it was mainly attributable to the local vasodilator effects of tissue hypoxia. 7. It is proposed that the components of the alerting response are an integral part of the response to systemic hypoxia. Further, that in the rat this response is superimposed upon, but may be overcome by the direct effects of hypoxia on peripheral vasculature, heart and central nervous system.
Full text
PDF




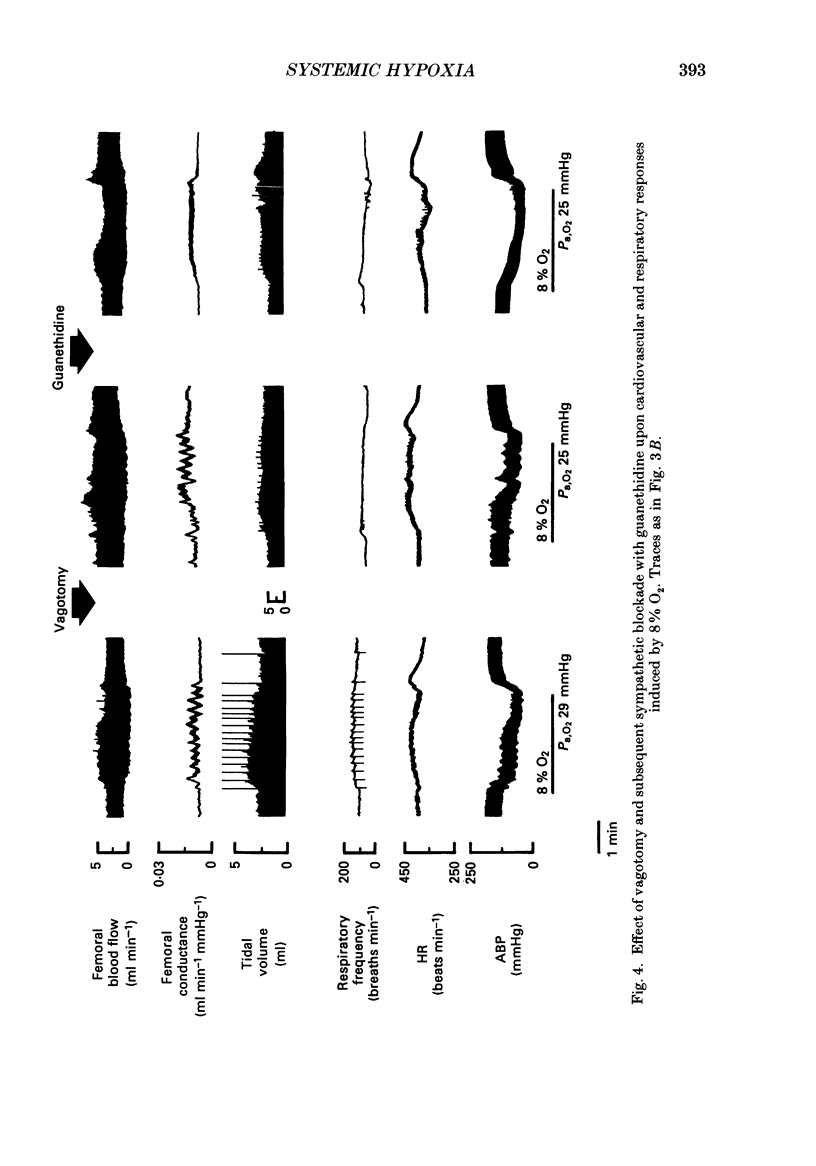









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHESON G. H., DAWES G. S., MOTT J. C. Oxygen consumption and the arterial oxygen saturation in foetal and new-born lambs. J Physiol. 1957 Mar 11;135(3):623–643. doi: 10.1113/jphysiol.1957.sp005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. P., Dieleman L. A., Cain S. M. A critical value for O2 transport in the rat. J Appl Physiol Respir Environ Exerc Physiol. 1982 Sep;53(3):660–664. doi: 10.1152/jappl.1982.53.3.660. [DOI] [PubMed] [Google Scholar]
- BLACK J. E., RODDIE I. C. The mechanism of the changes in forearm vascular resistance during hypoxia. J Physiol. 1958 Sep 23;143(2):226–235. doi: 10.1113/jphysiol.1958.sp006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop V. S., Thames M. D., Schmid P. G. Effects of bilateral vagal cold block on vasopressin in conscious dogs. Am J Physiol. 1984 Apr;246(4 Pt 2):R566–R569. doi: 10.1152/ajpregu.1984.246.4.R566. [DOI] [PubMed] [Google Scholar]
- Blanco C. E., Hanson M. A., Johnson P., Rigatto H. Breathing pattern of kittens during hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):12–17. doi: 10.1152/jappl.1984.56.1.12. [DOI] [PubMed] [Google Scholar]
- Daugherty R. M., Jr, Scott J. B., Dabney J. M., Haddy F. J. Local effects of O2 and CO2 on limb, renal, and coronary vascular resistances. Am J Physiol. 1967 Nov;213(5):1102–1110. doi: 10.1152/ajplegacy.1967.213.5.1102. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Forster H. V. Mediation of Ventilatory Adaptations. Physiol Rev. 1982 Jan;62(1):262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- Forsling M. L., Aziz L. A. Release of vasopressin in response to hypoxia and the effect of aminergic and opioid antagonists. J Endocrinol. 1983 Oct;99(1):77–86. doi: 10.1677/joe.0.0990077. [DOI] [PubMed] [Google Scholar]
- HILL J. R. The oxygen consumption of new-born and adult mammals. Its dependence on the oxygen tension in the inspired air and on the environmental temperature. J Physiol. 1959 Dec;149:346–373. doi: 10.1113/jphysiol.1959.sp006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton S. M., Marshall J. M. The pattern of cardiovascular response to carotid chemoreceptor stimulation in the cat. J Physiol. 1982 May;326:495–513. doi: 10.1113/jphysiol.1982.sp014208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler R. C., McDonald B. W., Krasney J. A. Influence of CO2 on cardiovascular response to hypoxia in conscious dogs. Am J Physiol. 1980 Oct;239(4):H545–H558. doi: 10.1152/ajpheart.1980.239.4.H545. [DOI] [PubMed] [Google Scholar]
- Mancia G., Donald D. E. Demonstration that the atria, ventricles, and lungs each are responsible for a tonic inhibition of the vasomotorcenter in the dog. Circ Res. 1975 Feb;36(2):310–318. doi: 10.1161/01.res.36.2.310. [DOI] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Cardiovascular changes associated with augmented breaths in normoxia and hypoxia in the rat. J Physiol. 1988 Jun;400:15–27. doi: 10.1113/jphysiol.1988.sp017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Tandon H. C. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984 May;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M. The role of the glycine sensitive area of the ventral medulla in cardiovascular responses to carotid chemoreceptor and peripheral nerve stimulation. Pflugers Arch. 1986 Feb;406(2):225–231. doi: 10.1007/BF00586687. [DOI] [PubMed] [Google Scholar]
- Rosén K. G., Kjellmer I. Changes in the fetal heart rate and ECG during hypoxia. Acta Physiol Scand. 1975 Jan;93(1):59–66. doi: 10.1111/j.1748-1716.1975.tb05790.x. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Blackmon J. R. Lack of sympathetic vasoconstriction in hypoxemic humans at rest. Am J Physiol. 1986 Sep;251(3 Pt 2):H562–H570. doi: 10.1152/ajpheart.1986.251.3.H562. [DOI] [PubMed] [Google Scholar]
- Sidi D., Kuipers J. R., Teitel D., Heymann M. A., Rudolph A. M. Developmental changes in oxygenation and circulatory responses to hypoxemia in lambs. Am J Physiol. 1983 Oct;245(4):H674–H682. doi: 10.1152/ajpheart.1983.245.4.H674. [DOI] [PubMed] [Google Scholar]
- Svanvik J., Tyllström J., Wallentin I. The effects of hypercapnia and hypoxia on the distribution of capillary blood flow in the denervated intestinal vascular bed. Acta Physiol Scand. 1968 Dec;74(4):543–551. doi: 10.1111/j.1748-1716.1968.tb04266.x. [DOI] [PubMed] [Google Scholar]
- Thimm F., Dienstel E., Meier zu Verl E. Heart rate changes caused by varying the oxygen supply to isolated hind legs of rats. Eur J Appl Physiol Occup Physiol. 1986;55(3):273–280. doi: 10.1007/BF02343799. [DOI] [PubMed] [Google Scholar]
- Uther J. B., Hunyor S. N., Shaw J., Korner P. I. Bulbar and suprabulbar control of the cardiovascular autonomic effects during arterial hypoxia in the rabbit. Circ Res. 1970 Apr;26(4):491–506. doi: 10.1161/01.res.26.4.491. [DOI] [PubMed] [Google Scholar]
- Yardley C. P., Hilton S. M. The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. J Auton Nerv Syst. 1986 Mar;15(3):227–244. doi: 10.1016/0165-1838(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Yardley C. P., Hilton S. M. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst. 1987 May;19(2):127–136. doi: 10.1016/0165-1838(87)90006-3. [DOI] [PubMed] [Google Scholar]


