Abstract
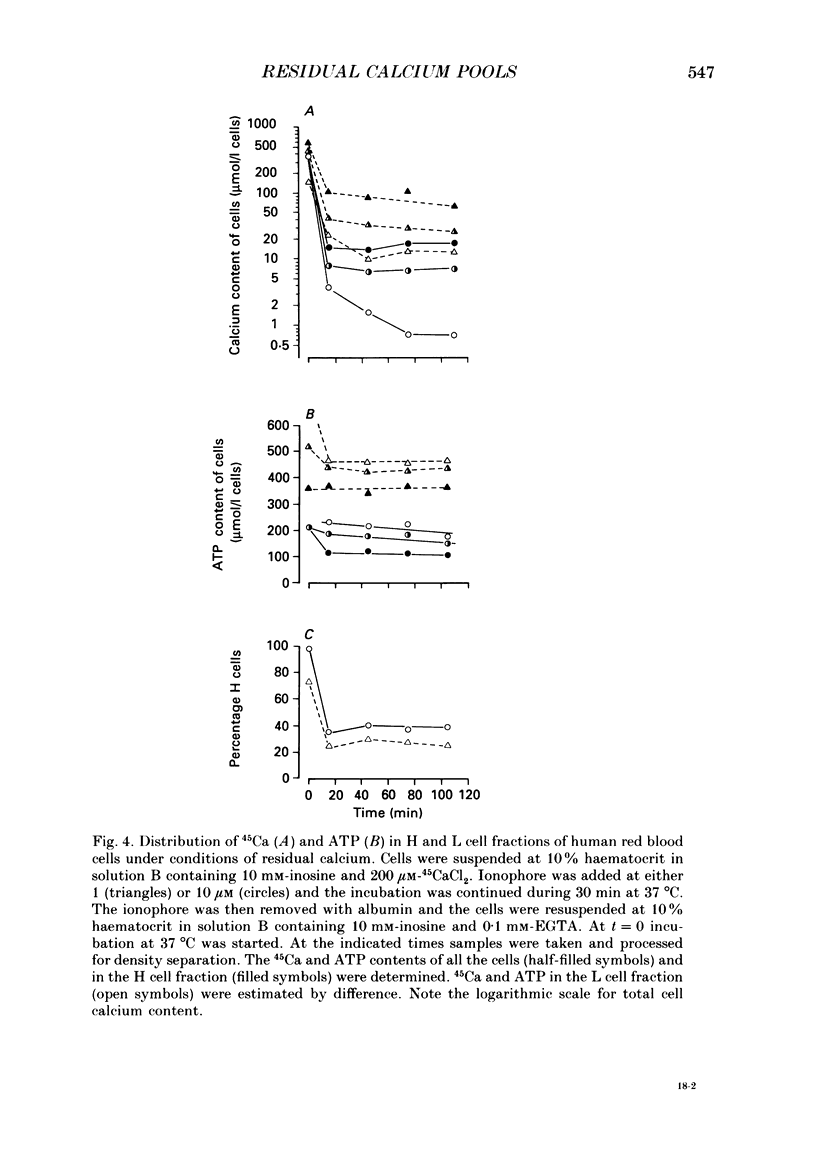
1. Inosine-fed human red cells, pre-loaded with calcium with the use of the ionophore A23187, and reincubated at 37 degrees C after ionophore and external calcium removal, pumped calcium out initially fast and then substantially slower when the residual calcium was still very high (García-Sancho & Lew, 1988 a, b). Particularly surprising was the finding of such residual calcium pools in a subpopulation of cells (L cells, García-Sancho & Lew, 1988b) with normal ATP, high active calcium pumping rates, and K+ channels which remained inactive during the density separation procedure (SCN- treatment; García-Sancho & Lew, 1988a). The purpose of the present experiments was to investigate the properties and possible origins of the residual calcium pools. 2. At each ionophore concentration, the pool size increased with the duration and magnitude of the calcium load. At comparable calcium loads, the pool size was smaller the higher the ionophore concentration used. 3. In cells from the same sample, residual calcium pools were present in cells that became dense after SCN- treatment (H cells) as well as those that remained in the light-cell fraction (L cells). Residual calcium was always higher in H cells than in L cells. 4. Re-exposure of cells to ionophore in Ca2+-free media could rapidly extract over 99% of their residual calcium. Residual calcium is therefore in a rapidly mobilizable form within a membrane-bound compartment. 5. Iodoacetamide-induced ATP depletion of H cells with high residual calcium-stimulated calcium loss. Such stimulation could only occur if ATP depletion inhibited a calcium-retaining process within the cells. 6. L cells with high residual calcium may have failed to dehydrate during SCN- treatment because of irreversible K+ channel inactivation or because K+ permeabilization would no longer generate a dehydrating net cation loss. These possibilities were tested and ruled out since it was found that virtually all cells became dense in low-K+, SCN- media after valinomycin addition or re-exposure to A23187 + calcium. 7. The results suggest that part if not all of the residual calcium is contained within compartments with the properties of endocytic inside-out vesicles capable of ATP-dependent calcium accumulation, such as those found in normal and abnormal human red cells (Lew, Hockaday, Sepúlveda, Somlyo, Somlyo, Ortiz & Bookchin, 1985).
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almaraz L., García-Sancho J., Lew V. L. Calcium-induced conversion of adenine nucleotides to inosine monophosphate in human red cells. J Physiol. 1988 Dec;407:557–567. doi: 10.1113/jphysiol.1988.sp017431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Lew V. L. Progressive inhibition of the Ca pump and Ca:Ca exchange in sickle red cells. Nature. 1980 Apr 10;284(5756):561–563. doi: 10.1038/284561a0. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Detection and separation of human red cells with different calcium contents following uniform calcium permeabilization. J Physiol. 1988 Dec;407:505–522. doi: 10.1113/jphysiol.1988.sp017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Heterogeneous calcium and adenosine triphosphate distribution in calcium-permeabilized human red cells. J Physiol. 1988 Dec;407:523–539. doi: 10.1113/jphysiol.1988.sp017429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. A Ca2+-refractory state of the Ca-sensitive K+ permeability mechanism in sickle cell anaemia red cells. Biochim Biophys Acta. 1980 Oct 16;602(1):196–200. doi: 10.1016/0005-2736(80)90301-6. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Hockaday A., Sepulveda M. I., Somlyo A. P., Somlyo A. V., Ortiz O. E., Bookchin R. M. Compartmentalization of sickle-cell calcium in endocytic inside-out vesicles. Nature. 1985 Jun 13;315(6020):586–589. doi: 10.1038/315586a0. [DOI] [PubMed] [Google Scholar]
- Ortiz O. E., Lew V. L., Bookchin R. M. Calcium accumulated by sickle cell anemia red cells does not affect their potassium (86Rb+) flux components. Blood. 1986 Mar;67(3):710–715. [PubMed] [Google Scholar]
- Simons T. J. A method for estimating free Ca within human red blood cells, with an application to the study of their Ca-dependent K permeability. J Membr Biol. 1982;66(3):235–247. doi: 10.1007/BF01868498. [DOI] [PubMed] [Google Scholar]
- Tiffert T., Garcia-Sancho J., Lew V. L. Irreversible ATP depletion caused by low concentrations of formaldehyde and of calcium-chelator esters in intact human red cells. Biochim Biophys Acta. 1984 Jun 13;773(1):143–156. doi: 10.1016/0005-2736(84)90559-5. [DOI] [PubMed] [Google Scholar]


