Abstract
1. The uphill calcium efflux through calcium-saturated pumps in intact red cells was investigated with the aid of a new method, in initial conditions of uniform ionophore A23187-induced calcium distribution among the cells. The method is based on findings by Tiffert, García-Sancho & Lew (1984) which show that cobalt can suddenly arrest passive calcium transport by the ionophore and expose, without noticeable interference, uphill calcium extrusion by the pump. The results comprise methodological aspects and questions concerning interactions between inner pump sites, ATP and Ca2+, and the calcium: ATP stoichiometry of the calcium-saturated pump. 2. Ionophore-induced calcium influx was set to be far in excess of the maximal calcium pump capacity. This secured a uniform calcium distribution among the cells, and Ca2+ equilibration by 2 min or less of calcium permeabilization. Cobalt was added between 15 s and 5 min after ionophore addition. The calcium and ATP content of the cells was followed during ionophore-induced influx and cobalt-exposed efflux. 3. The external cobalt concentrations required to block completely ionophore-mediated calcium transport were similar or only marginally higher than those of calcium. 4. The reproducibility of independent cobalt-exposed calcium efflux measurements from single blood samples was within an 8% range. 5. During cobalt-exposed calcium efflux, the calcium content of subpopulations of cells, with and without active Ca2+-sensitive K+ channels, investigated by post-incubation of samples in low-K+, thiocyanate (SCN-) media (modified from García-Sancho & Lew, 1988a), was similar. This is consistent with the maintenance of a uniform calcium distribution among the cells during uphill calcium extrusion. 6. Cobalt-exposed calcium efflux was similar in the interval from 15 s to 5 min after calcium permeabilization although cell ATP levels had fallen by over 50% in that period. Therefore, cell ATP concentrations within the physiological range do not seem to be regulatory for calcium-saturated pumps in the intact red cell. 7. All cobalt-exposed calcium efflux curves were linear in time, at least until total cell calcium contents reached levels below 100 mumol/l cells. This suggests that internal calcium is not inhibitory for calcium-saturated efflux in intact cells in the 0.1-1 mmol/l cells range. 8. The cobalt-exposed calcium fluxes were in the range from 4 to 24 mmol/(1 cells.h) for fresh cells and from 10 to 18 mmol/1 cells. h) for samples from the Blood Bank.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



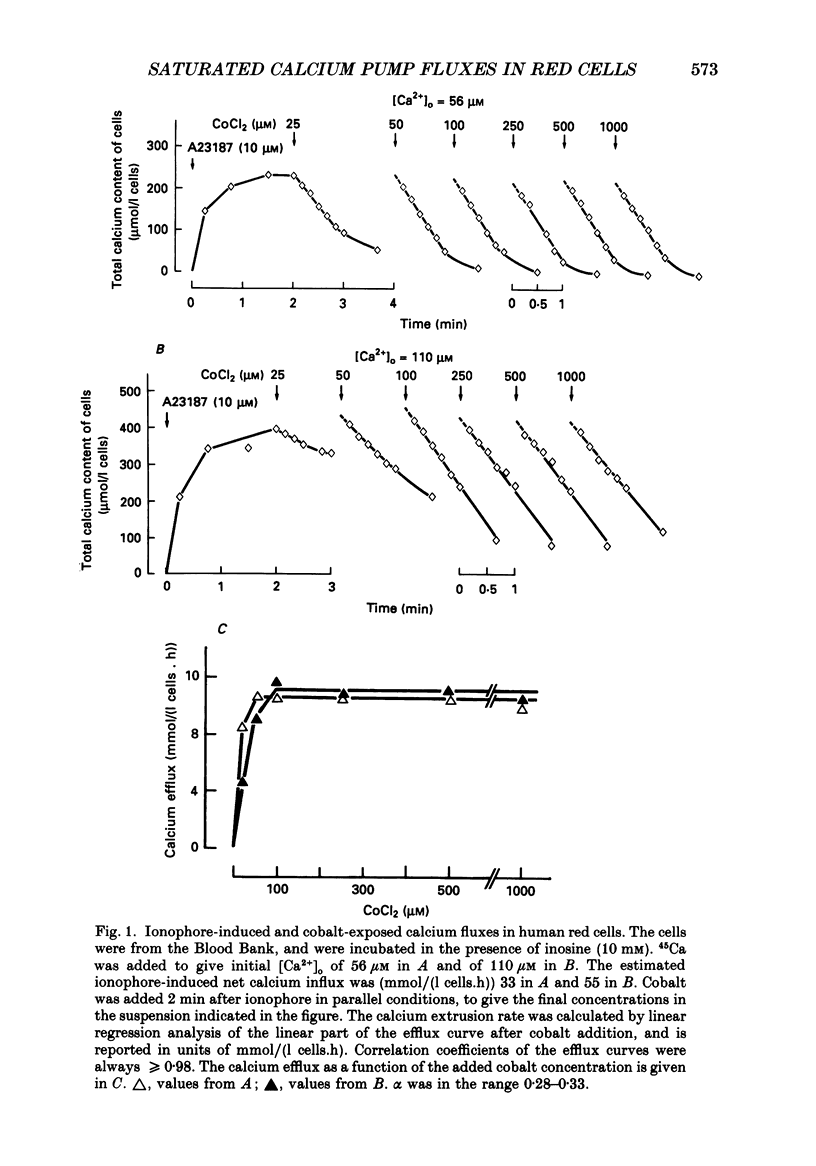
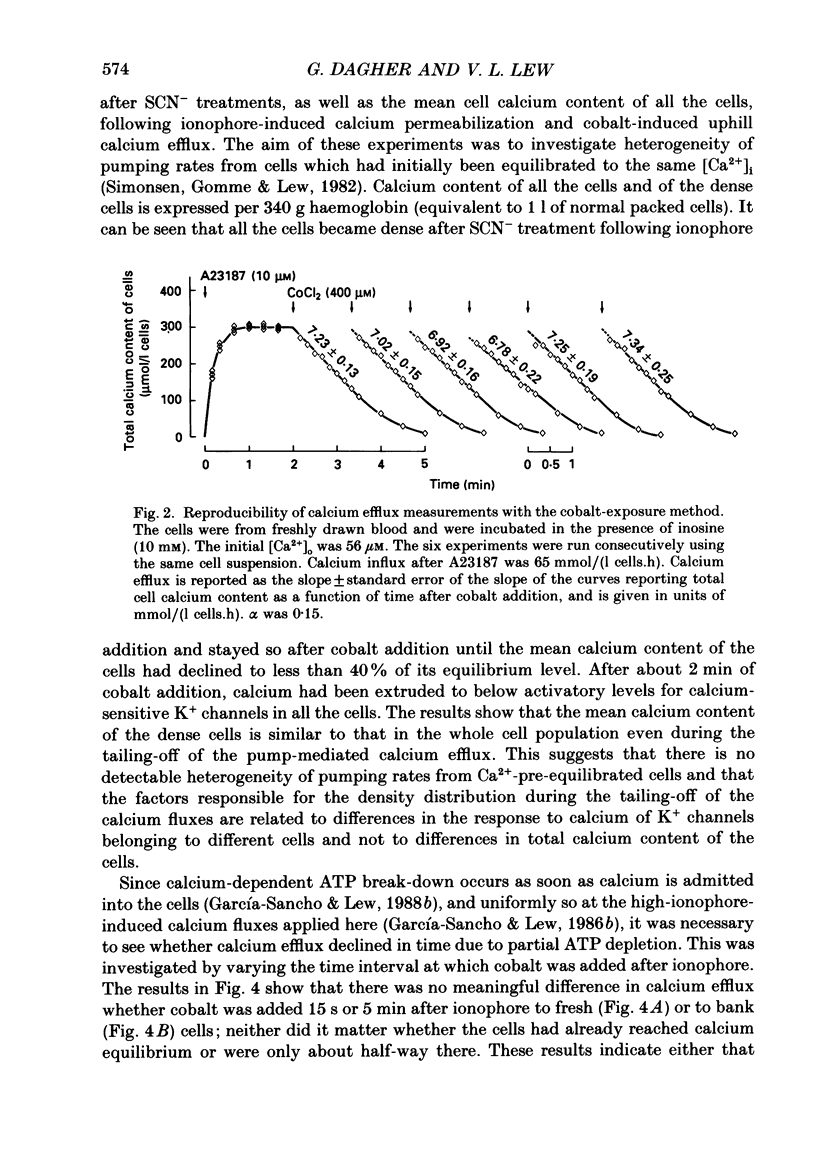
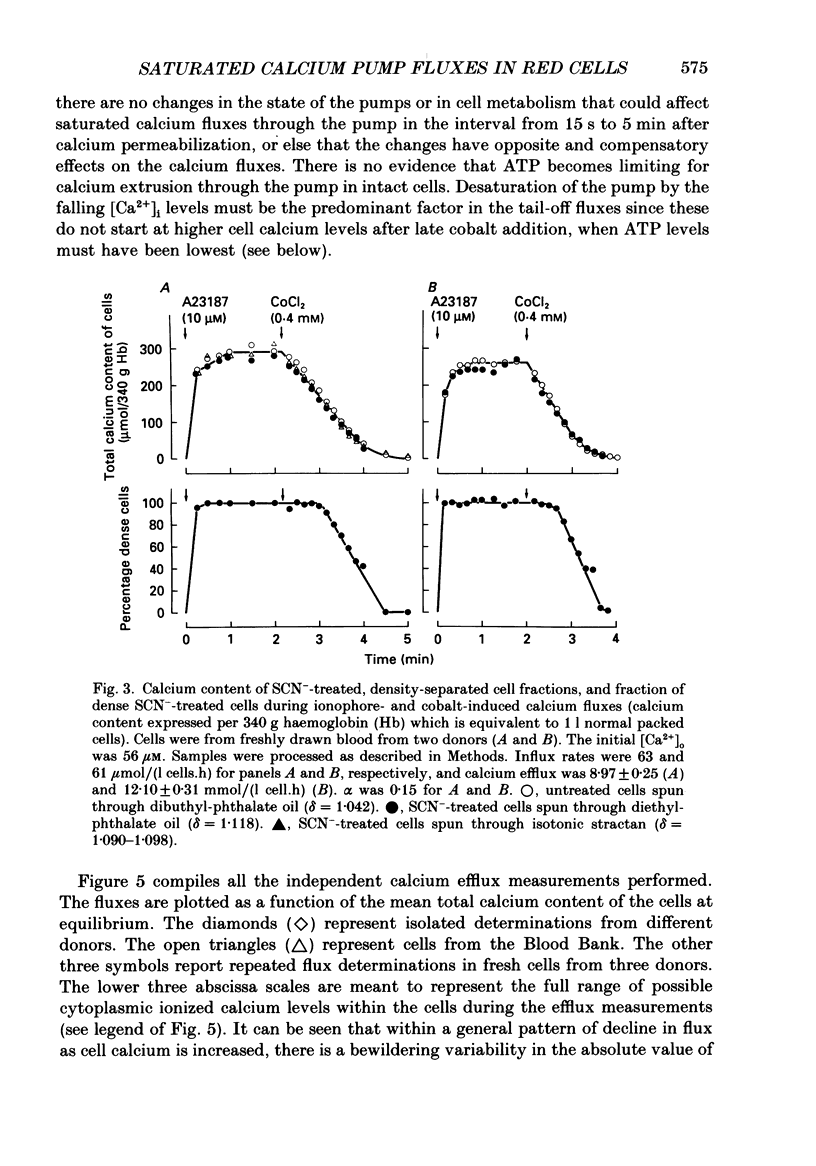
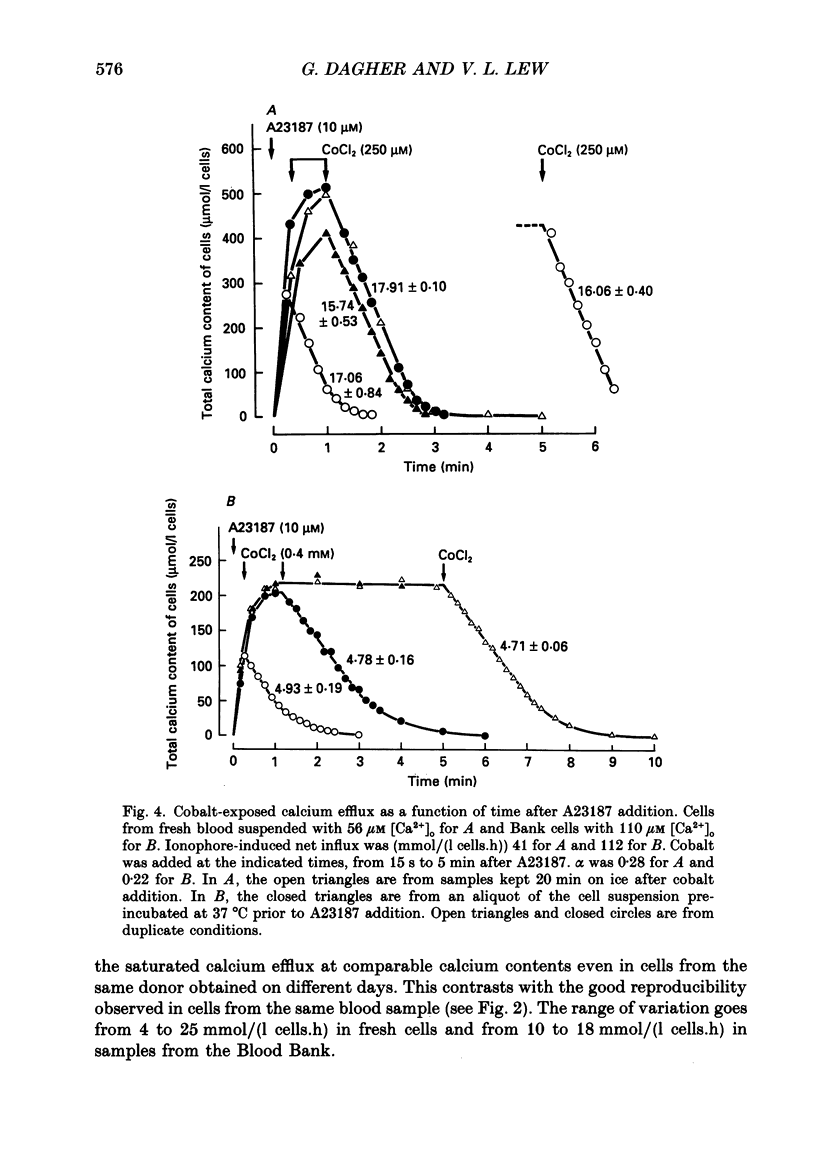
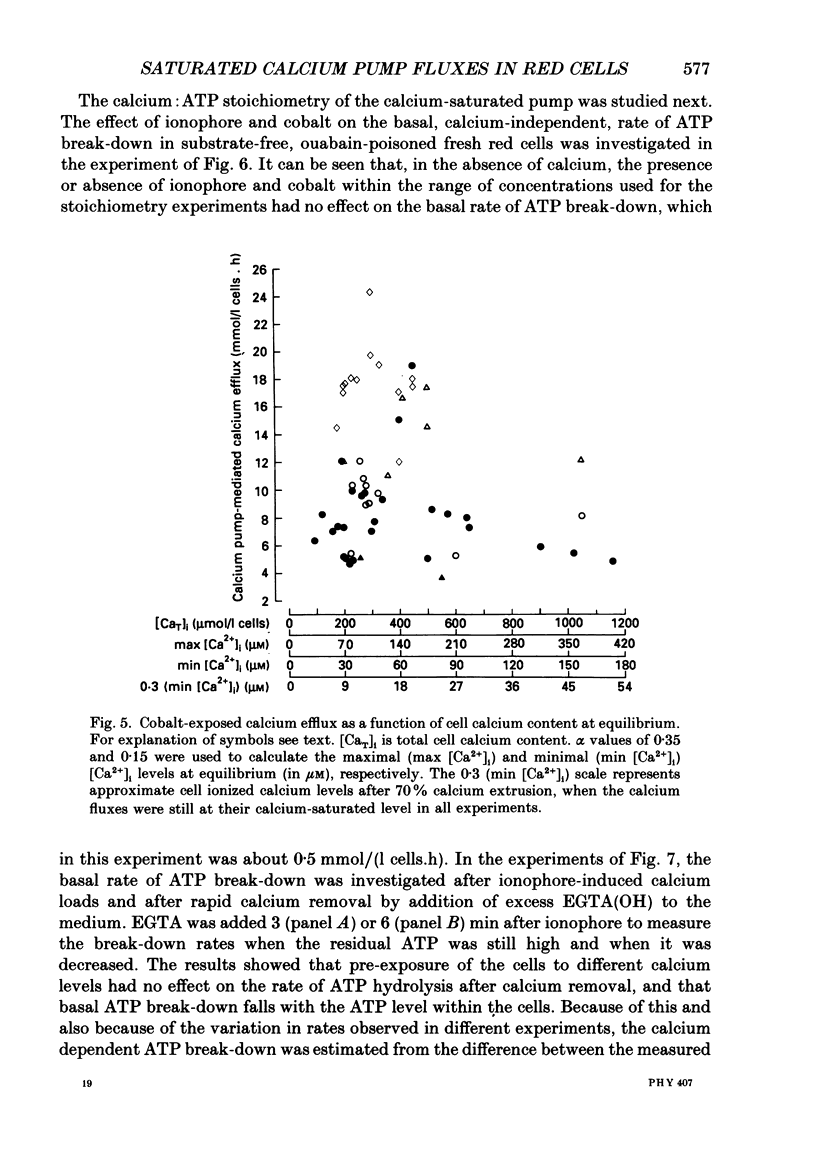
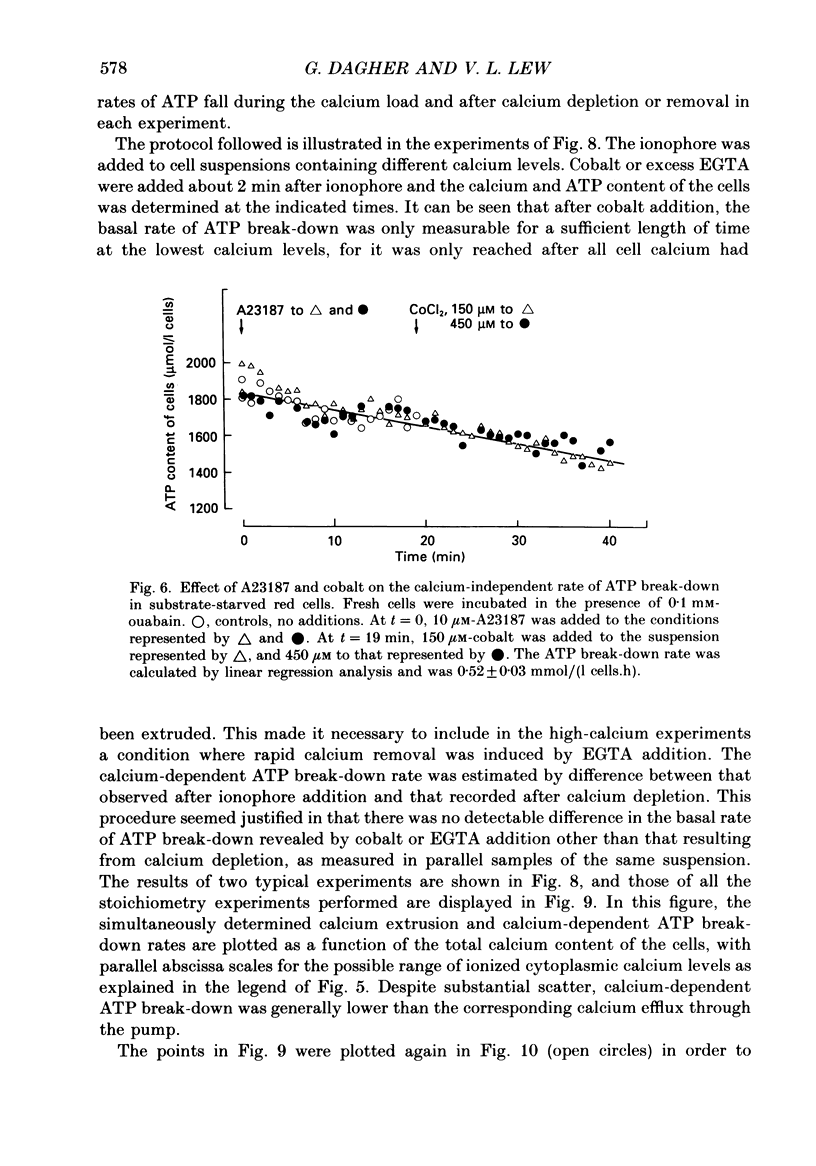
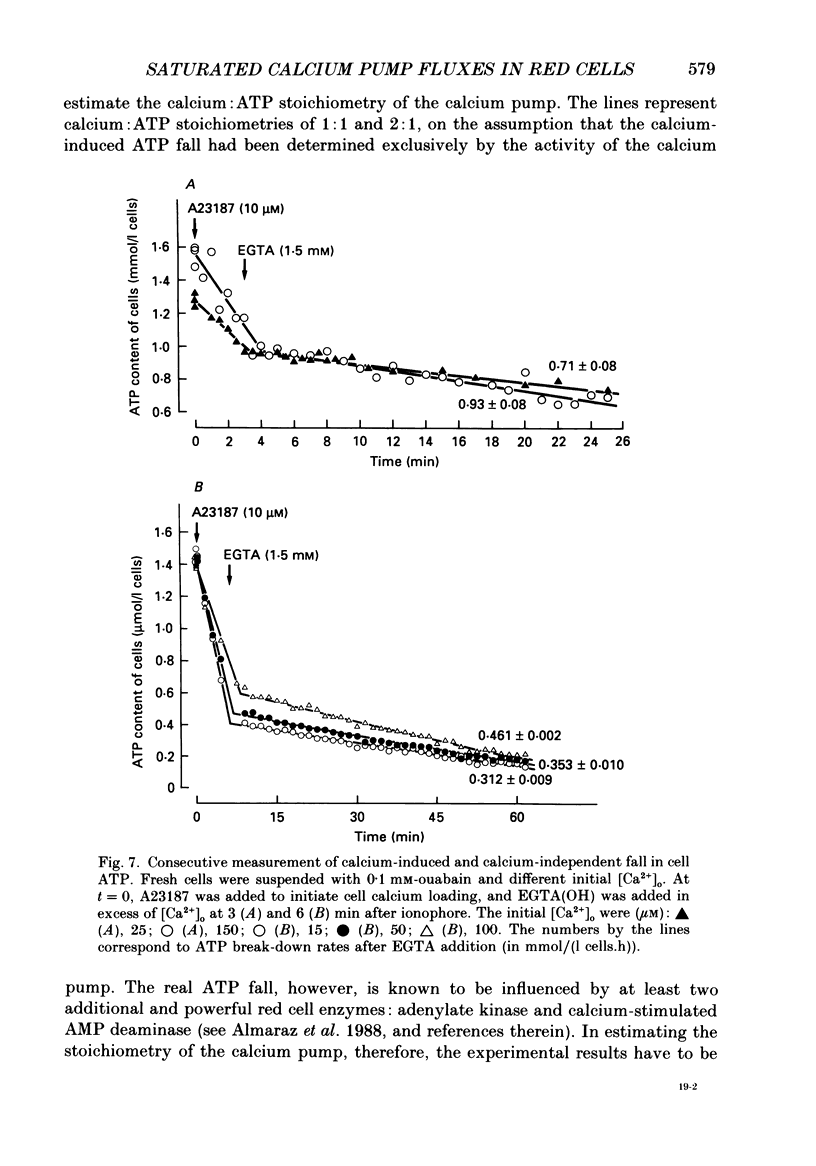
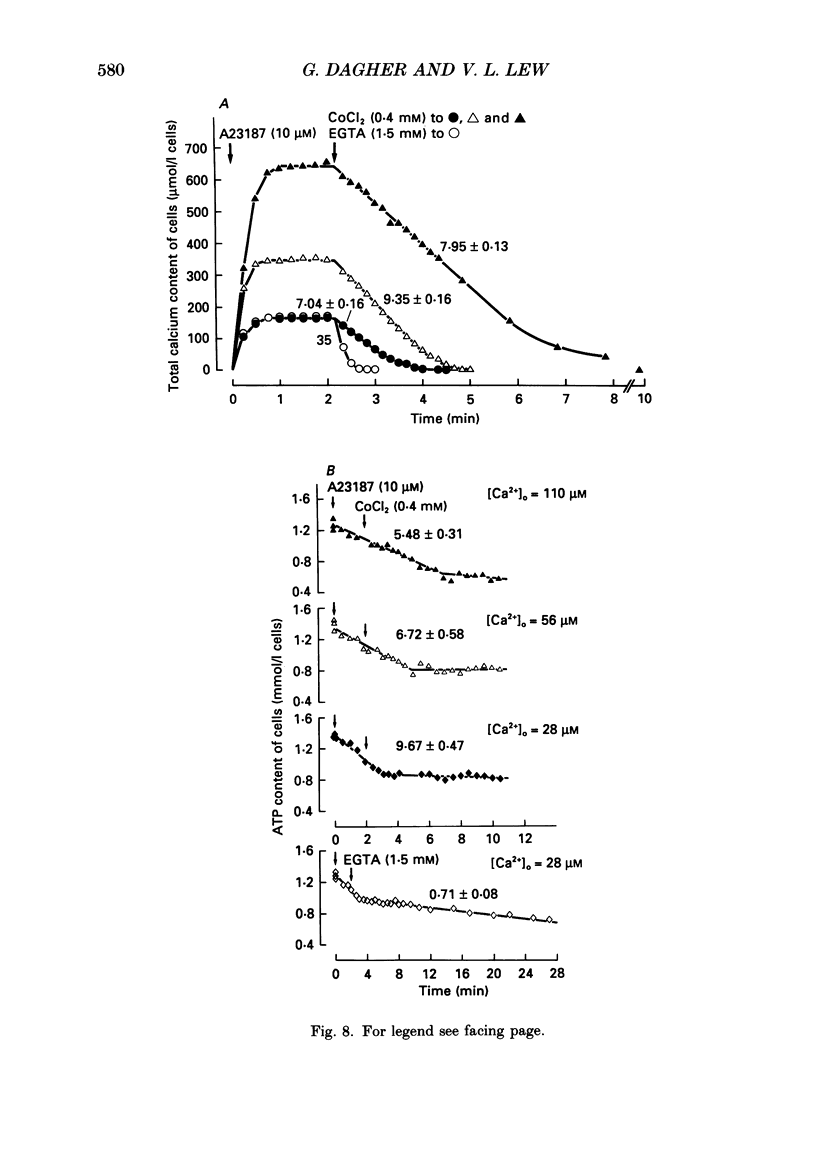
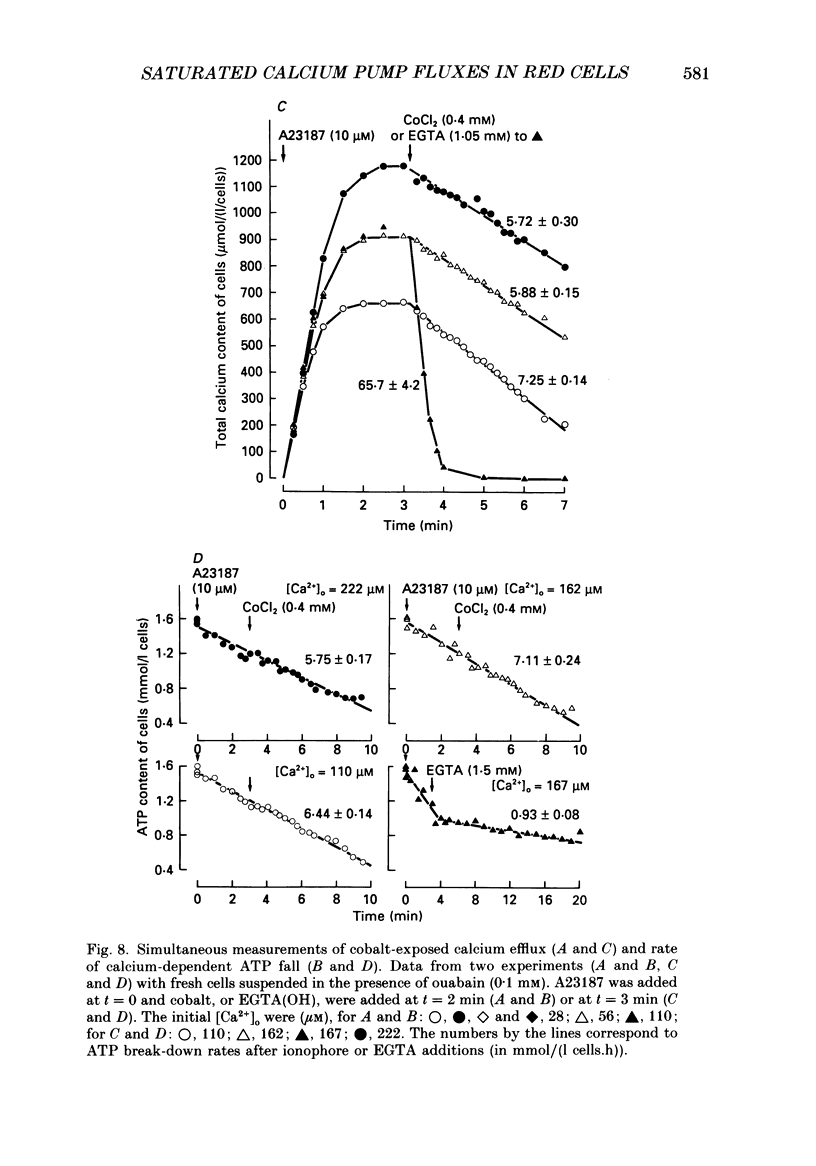
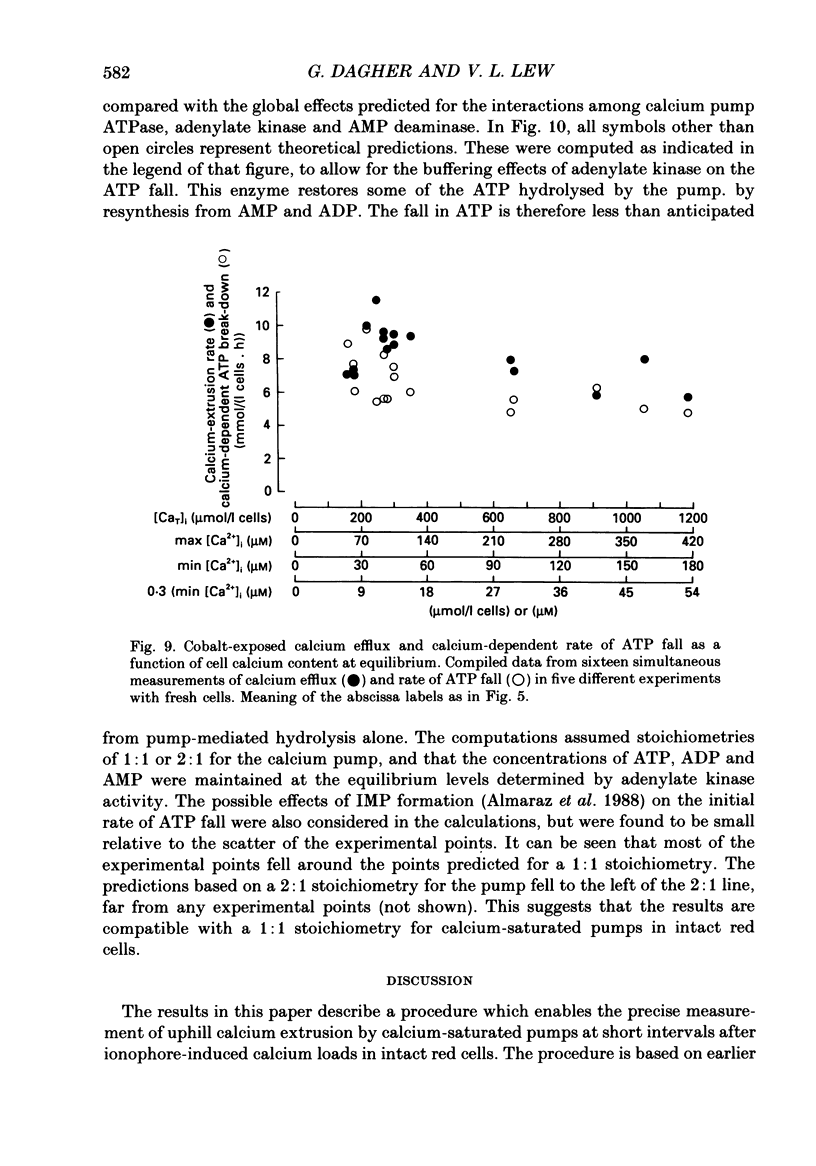
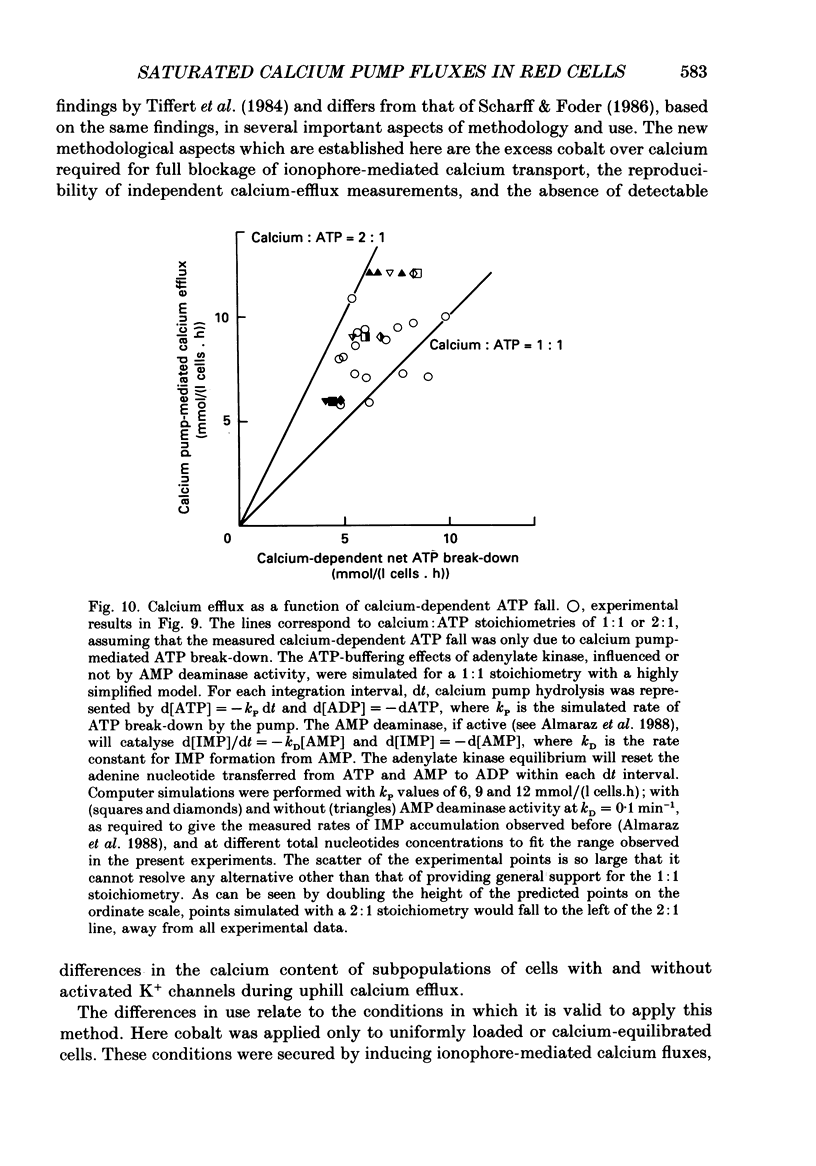



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jobore A., Minocherhomjee A. M., Villalobo A., Roufogalis B. D. Active calcium transport in normal and abnormal human erythrocytes. Prog Clin Biol Res. 1984;159:243–292. [PubMed] [Google Scholar]
- Almaraz L., García-Sancho J., Lew V. L. Calcium-induced conversion of adenine nucleotides to inosine monophosphate in human red cells. J Physiol. 1988 Dec;407:557–567. doi: 10.1113/jphysiol.1988.sp017431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Lew V. L. Progressive inhibition of the Ca pump and Ca:Ca exchange in sickle red cells. Nature. 1980 Apr 10;284(5756):561–563. doi: 10.1038/284561a0. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Detection and separation of human red cells with different calcium contents following uniform calcium permeabilization. J Physiol. 1988 Dec;407:505–522. doi: 10.1113/jphysiol.1988.sp017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Heterogeneous calcium and adenosine triphosphate distribution in calcium-permeabilized human red cells. J Physiol. 1988 Dec;407:523–539. doi: 10.1113/jphysiol.1988.sp017429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Properties of the residual calcium pools in human red cells exposed to transient calcium loads. J Physiol. 1988 Dec;407:541–556. doi: 10.1113/jphysiol.1988.sp017430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Activation of partial reactions of the Ca2+-ATPase from human red cells by Mg2+ and ATP. Biochim Biophys Acta. 1978 Oct 19;513(1):59–65. doi: 10.1016/0005-2736(78)90111-6. [DOI] [PubMed] [Google Scholar]
- Kratje R. B., Garrahan P. J., Rega A. F. Two modes of inhibition of the Ca2+ pump in red cells by Ca2+. Biochim Biophys Acta. 1985 Jun 27;816(2):365–378. doi: 10.1016/0005-2736(85)90504-8. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Garcia-Sancho J. Use of the ionophore A23187 to measure and control cytoplasmic Ca2+ levels in intact red cells. Cell Calcium. 1985 Apr;6(1-2):15–23. doi: 10.1016/0143-4160(85)90031-4. [DOI] [PubMed] [Google Scholar]
- Lichtner R., Wolf H. U. Phosphorylation of the isolated high-affinity (Ca2+ + Mg2+) ATPase of the human erythrocyte membrane. Biochim Biophys Acta. 1980 Jun 6;598(3):472–485. doi: 10.1016/0005-2736(80)90028-0. [DOI] [PubMed] [Google Scholar]
- Mualem S., Karlish S. J. Is the red cell calcium pump regulated by ATP? Nature. 1979 Jan 18;277(5693):238–240. doi: 10.1038/277238a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Catalytic and regulatory ATP-binding sites of the red cell Ca2+ pump studied by irreversible modification with fluorescein isothiocyanate. J Biol Chem. 1983 Jan 10;258(1):169–175. [PubMed] [Google Scholar]
- Richards D. E., Rega A. F., Garrahan P. J. Two classes of site for ATP in the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1978 Aug 4;511(2):194–201. doi: 10.1016/0005-2736(78)90313-9. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Rega A. F., Garrahan P. J. Compound 48/80 and calmodulin modify the interaction of ATP with the (Ca2+ + Mg2+)-ATPase of red cell membranes. Biochim Biophys Acta. 1985 Jun 27;816(2):379–386. doi: 10.1016/0005-2736(85)90505-x. [DOI] [PubMed] [Google Scholar]
- Sarkadi B. Active calcium transport in human red cells. Biochim Biophys Acta. 1980 Sep 30;604(2):159–190. doi: 10.1016/0005-2736(80)90573-8. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Delayed activation of calcium pump during transient increases in cellular Ca2+ concentration and K+ conductance in hyperpolarizing human red cells. Biochim Biophys Acta. 1986 Oct 23;861(3):471–479. doi: 10.1016/0005-2736(86)90456-6. [DOI] [PubMed] [Google Scholar]
- Simonsen L. O., Gomme J., Lew V. L. Uniform ionophore A23187 distribution and cytoplasmic calcium buffering in intact human red cells. Biochim Biophys Acta. 1982 Nov 22;692(3):431–440. doi: 10.1016/0005-2736(82)90394-7. [DOI] [PubMed] [Google Scholar]
- Tiffert T., Garcia-Sancho J., Lew V. L. Irreversible ATP depletion caused by low concentrations of formaldehyde and of calcium-chelator esters in intact human red cells. Biochim Biophys Acta. 1984 Jun 13;773(1):143–156. doi: 10.1016/0005-2736(84)90559-5. [DOI] [PubMed] [Google Scholar]


