Abstract
Covalent organic frameworks (COFs) have become a promising class of highly-crystalline polymers with layered stacking structures, ordered porous channels, and highly-tailorable structures. To date, most COFs have been synthesized via high-temperature solvothermal methods, which require complicated optimization of factors including temperature, solvent ratio, catalyst, and reaction time. Additionally, solvothermal conditions with high temperature and high pressure restrict the facile and large-scale synthesis of COFs for practical applications. In addition, the insolubility and lack of processability of the COF powders obtained via solvothermal methods hinder their potential application in film-related fields. Energy-efficient and environmentally benign synthetic methods to resolve these problems are highly desired. In this review, we provide an overview of the recent progress in room-temperature synthetic strategies for constructing COF powders or COF films. We first discuss in situ characterization technologies for exploring the COF growth mechanism. Then, we present representative room-temperature synthesis methods for COFs, including solid–liquid interfacial synthesis, liquid–liquid interfacial synthesis, on-water surface synthesis, water-phase synthesis, electrosynthesis, sonochemical synthesis, single-solution phase synthesis, mechanochemical synthesis, high-energy ionizing radiation synthesis, and photochemical synthesis. Finally, perspectives on room-temperature synthesis are proposed in the areas of single-crystal domains, novel room-temperature reaction types, crystallization mechanism, the design of chemical structures and green synthesis.
Room-temperature synthesis bridges the gap between the chemistry and practical application of COFs. This review provides an overview of the characterization technologies of COF growth mechanisms and recent room-temperature synthetic strategies.
1. Introduction
Covalent organic frameworks (COFs) are a new class of highly crystalline organic polymers with highly adjustable structures and properties, in which the monomers and scaffolds are precisely arranged in two-dimensional (2D) or three-dimensional (3D) space.1 The 2D layered COF structures are formed through van der Waals interactions between 2D planes. Dynamic covalent chemistry is considered to be the chemical basis by which the reversible chemical bonds can realize self-healing of internal defects in COF materials.2 Recently, some irreversible reactions have also been used to synthesize COFs,3 which requires more effort to be devoted to the structural design and the study of crystallization mechanism. However, to improve the crystallinity of COFs, tedious solvent screening, high reaction temperatures (>120 °C) and lengthy polymerization times (>3 days) are always selected for the optimized solvothermal synthesis.4–6 The resulting time-consuming and energy-intensive solvothermal syntheses make the large-scale production of COFs challenging.
In terms of determining the crystallization mechanism, the use of closed solvothermal reaction systems hinders in situ characterization of the dynamic process.7–9 The heat source and sealed conditions limit the development of in situ monitoring equipment. Thus, in most reports involving new COF structures, solvothermal synthesis has been the first choice, and less attention has been paid to the crystallization mechanism.10–12 Additionally, COFs synthesized by the solvothermal method are insoluble and unprocessable powders, which present formidable challenges in film-based applications.13–15
In the exploration of alternative synthetic approaches, the room-temperature synthesis of COFs has attracted increasing interest.16–18 Compared with harsh solvothermal syntheses, room-temperature syntheses without a high-pressure environment feature mild reaction conditions, designable reaction systems, various assisted technologies and controllable reaction rate. Currently, various condensation reactions have been used to synthesize COFs at room temperature, such as the Suzuki coupling reaction, Schiff-base reaction, boronate ester condensation, Knoevenagel condensation, Mannich reaction, multicomponent reactions and imide reactions. In addition, unlike the polycrystalline products obtained via solvothermal syntheses, large-size single-crystal COFs can be prepared via low-temperature or room-temperature syntheses.19–21 The growth of defects and single crystallinity can be well regulated and controlled via designed room-temperature syntheses.
In 2012, sonochemistry was first used for the rapid room-temperature synthesis of COFs. Subsequently, various advanced synthetic methods have been created for preparing COFs at room temperature. As shown in Fig. 1, in the last decade, various pioneering room-temperature synthetic strategies have been reported, such as solid–liquid interfacial synthesis, liquid–liquid interfacial synthesis, on-water surface synthesis, water-phase synthesis, electrosynthesis, sonochemical synthesis, single-solution phase synthesis, mechanochemical synthesis, high-energy ionizing radiation synthesis, and photochemical synthesis. In addition, to explore the crystallization mechanism, various in situ characterization technologies have been designed and used. In this review, with the aim of promoting innovations in the preparation of COF materials and their practical applications, a timely overview of the room-temperature synthesis of COFs is provided.
Fig. 1. Schematic presenting a timeline of the development of the room-temperature synthesis of COFs.
2. In situ characterization technologies for exploring the COF growth mechanism
The study of the crystallization mechanism of COFs is of great significance for developing new synthetic strategies. Ex situ/in situ characterization technologies have been used to investigate their growth mechanisms. Unlike ex situ characterization technologies for observing information after the experimental process, in situ characterization technologies can monitor the changes during the experimental process directly.22
2.1. IR spectroscopy
Infrared (IR) spectroscopy can record changes in the micro-environment and linkages via the location and intensity of the infrared absorption peaks of functional groups. Ex/in situ IR spectroscopy can monitor the dynamic evolution of linkages during the synthesis of COFs. Banerjee and co-workers investigated the dynamic evolution of C O and NH2 groups via time-dependent IR during the covalent self-assembly of COF nanospheres (Fig. 2a).23 Over 36 h, the –C O peak at 1719 cm−1 and two –NH2 peaks at 3324 cm−1 and 3354 cm−1 of the COF nanospheres gradually disappeared in the time-dependent IR spectra of a TpAzo COF film, indicating the end of the covalent self-assembly.
Fig. 2. (a) Time-dependent IR of the formation of a COF film. Reproduced with permission from ref. 23. Copyright 2019 American Chemical Society. (b) In situ Raman spectroscopy during the synthesis of COF-1. (c) The scatter data of COF-1 and PDA. Reproduced with permission from ref. 26. Copyright 2024 Elsevier Ltd (d) 2D XRD plots during the mechanochemical reaction of COF-LZU1. (e) Normalized integrated peak intensities of COF-LZU1 and intermediates. Reproduced with permission from ref. 28. Copyright 2024 Wiley-VCH. (f) Real-time crystallization monitoring via in situ TEM during COF onion formation at 60 °C in liquid. Reproduced with permission from ref. 32. Copyright 2024 American Chemical Society.
2.2. Raman spectroscopy
Raman spectroscopy is typically used to determine the vibrational modes of molecules.24 Raman spectroscopy and IR are complementary in the study of linkages.25 COFs with symmetric molecular structures can be easily monitored using Raman techniques. Thus, the changes from disorder to order and the evolution from monomers to COFs can be detected using Raman spectroscopy. Recently, to investigate the evolution of linkages during mechanochemical synthesis, in situ Raman spectroscopy has been used to reveal the formation of C N or boroxine ring linkages and the depletion of monomers.26,27 As shown in Fig. 2b and c, Perepichka and co-workers provided the first in situ Raman spectroscopic insight into boronic acid condensation during the mechanochemical synthesis of COF-1; the peak intensity of the boroxine ring at 673 cm−1 gradually increased as the boronic acid peak at 1100 cm−1 gradually disappeared over the course of 3 h.26
2.3. X-ray diffraction
X-ray diffraction (XRD) is an important tool to study the structural information of crystals. The evolution of crystal faces can be detected via the location and intensity of the XRD peaks. Generally, ex situ XRD has been used to study the effects of different reaction conditions on the crystallinity. Recently, Lotsch and co-workers used in situ XRD and in situ Raman to monitor reaction intermediates during the mechanochemical synthesis of COF-LZU-1.28 As shown in Fig. 2d and e, the XRD images of the intermediates were different from those of the monomers. Over the 50 min reaction, obvious strengthening of the (100) plane of COF-LZU1 with decreasing intensity of intermediates were clearly observed. The in situ studies offered direct experimental evidence of 1,4-dioxane templating effects based on the observation of the solvated reaction intermediates.
2.4. Microscopy
Microscopy technologies, including transmission electron microscopy (TEM) and scanning electron microscopy (SEM), can be used to observe morphological changes and crystalline structure.29 Due to the limitations of in situ reaction equipment, time-dependent ex situ TEM and SEM have typically been adopted by researchers to monitor morphology and structure.30,31 Very recently, using in situ liquid-phase TEM, Zheng and co-workers elucidated the multistep pathways of COF onion nanostructure formation, as demonstrated in Fig. 2f. Real-time images acquired over 150 s via in situ TEM directly captured the graphitic layer formation, interlayer attachment, spacing relaxation, and structural homogenization.32 The direct evidence of crystallization provided unprecedented insights into the evolution of the crystallinity.
3. Room-temperature synthetic strategies for COFs
3.1. Solid–liquid interfacial synthesis
In the last decade, substrate-assisted interface polymerization has been performed to synthesize COF films via high-temperature solvothermal methods.33 However, the harsh reaction conditions and limited volume of the reaction vessel hinder the preparation of large-scale films and lower the film quality. Thus, the solid–liquid interface synthesis of COF films at room temperature, which can enhance the film quality by lowering the reversible reaction rate and achieve large-scale COF films using simple reaction equipment, has been explored.34,35
In 2015, Bein and co-workers developed a mild substrate- and vapor-assisted conversion for growing boronic-ester-linked COF films on glass substrates.36 First, the COF precursors were dissolved in a polar solvent mixture. The mixture was then dripped onto a clean glass substrate. To ensure complete evaporation of the solvent, the wet glass substrate was placed in a desiccator containing methylene and dioxane (v/v = 1 : 1) at room temperature for 72 h. Finally, a dark green COF film was obtained on the glass. In addition, Lai and co-workers synthesized a highly crystalline COF film (COF-DT) at room temperature via placing an SiO2/Si substrate in a dilute precursor solution of 2,5-dihydroxy-1,4-phenyldialdehyde (DHTA) and 1,3,5-tri(4-aminophenyl)benzene (TAPB) for solid–liquid interfacial growth.37 As shown in Fig. 3a, the diluted solution allowed for slow nucleation and direct growth of the COF film. After 48 h, the SiO2/Si substrate was removed to obtain a COF-DT film, which was transferred to a PET carrier. Similarly, Chen and co-workers also reported in situ grown COF films via a solid–liquid interface in a dilute precursor solution.38 The substrates were placed vertically into a precursor solution with a very low initial monomer concentration. As expected, heterogeneous nucleation occurred on the substrate surface. The in situ grown COF films had high crystallinity, uniform morphology and controllable thickness.
Fig. 3. (a) Solid–liquid interfacial synthesis of COF-DT films. Reproduced with permission from ref. 37. Copyright 2022 Wiley-VCH. (b) Fabrication of COF films at liquid–liquid and solid–liquid interfaces via interfacial and residual crystallization. Reproduced with permission from ref. 39. Copyright 2021 American Chemical Society.
Interestingly, Banerjee and co-workers reported a novel residual crystallization (RC) method (Fig. 3b).39 Different from the above method, a two-phase liquid–liquid system was designed, and the substrate was preplaced in the lower phase to form a solid–liquid interface. During the interfacial reactions, most of the crystallization reaction occurs at the liquid–liquid interface, and only a trace amount of residue crystallizes at the solid–liquid interface to form a COF film. The COF thin films synthesized via the RC method showcased high surface area, crystallinity, and conductivity at room temperature. A similar experimental method also was demonstrated by Alshareef and co-workers.40
3.2. Liquid–liquid interfacial synthesis
Liquid–liquid interfacial synthesis requires a space-confined interface to grow 2D COF films. There are two general kinds of interfacial designs: (1) two kinds of monomers are dispersed in two different phases, which drives the dynamic reversible reaction to occur at the interface of the two phases. (2) The reactant and catalyst are dispersed in two different phases, ensuring that the catalytic reaction occurs between the two phases. The liquid/liquid interface is usually constructed using two immiscible liquids or two immiscible liquids plus a buffer interlayer, which confines the reactions to the 2D interface to form COF films. Common liquid–liquid two-phase interfaces include water/dichloromethane (DCM),30 water/ionic liquid,41 water/nitrobenzene42 and water/ethyl acetate interfaces.43
In 2017, Banerjee and co-workers reported organic salt-mediated liquid–liquid interface synthesis technology, in which hydrogen bonding between an ammonium salt and water in aqueous solution can slow its diffusion rate; the slow interfacial diffusion rate drives the reaction in the direction of thermodynamically controlled crystallization (Fig. 4a).31 Typically, 1,3,5-triformyl-phloroglucinol (Tp) was dissolved in DCM, and a water layer was then added on the DCM solution as a buffer layer to form a water/oil interface. An aqueous solution of dissolved aromatic amine-p-toluenesulfonic acid (PTSA) salts was slowly added to the buffer layer. After 72 h at room temperature, the self-supporting COF film was formed. Recently, Liu and co-workers reported the oil-in-water (O/W) emulsion interface-confined synthesis of imine-based 2D covalent organic frameworks (COFs) stabilized by cationic surfactants.44 In addition to Schiff base reactions, the liquid–liquid interface technique can also be extended to the Suzuki coupling reaction45 and Knoevenagel reaction.46 In 2018, Li and co-workers synthesized carbon–carbon-bonded COF films through the Suzuki coupling reaction in a water/toluene two-phase system, in which the toluene phase contained reaction monomers and the catalyst Pd(PPh3)4 was dropped on top of the K2CO3 aqueous solution.45 After a month of reaction in a 2 °C refrigerator under an argon atmosphere, 2D COF films with a large transverse size and crystal domain were obtained. Dong and co-workers used a different type of catalyst system with toluene-soluble bis(tert-butylphosphine)palladium(0) and water-soluble NaOH as the catalysts to prepare 2D conjugated polymers with carbon–carbon bonds at the interface between toluene and water (Fig. 4b). The reaction was completed in a relatively short time.47
Fig. 4. (a) Schematic representation of the liquid–liquid interfacial crystallization process for synthesizing the Tp-Bpy thin film. Reproduced with permission from ref. 31. Copyright 2017 American Chemical Society. (b) Schematic of synthesis of a 2DPTTI film through a Suzuki reaction between the monomers 2-BrTTI and BADE. Reproduced with permission from ref. 47. Copyright 2020 Wiley-VCH.
3.3. On-water surface synthesis
Unlike liquid–liquid interface syntheses, on-water surface synthesis methods can effectively prepare ultra-thin or even single-layer 2D COFs, the Langmuir–Blodgett (LB) method and surfactant-monolayer-assisted interfacial synthesis are the main on-water surface synthesis methods.
Hu and co-workers synthesized a series of single-layer 2D polymers at the air–water interface via LB methods (Fig. 5a).48,49 In the LB method, very small amounts of monomers are needed, and the surface pressure can be precisely controlled. Typically, the monomers were dissolved in a volatile organic solvent such as chloroform or dichloromethane. The LB trough was first filled with deionized water. Then, a mixed solution of monomers was carefully added to the water sub-phase using a micro-syringe. The volatile organic solvent was allowed to evaporate for 30 min, and then compression was performed at a rate of 2.98 mm min−1 until the surface pressure reached 3 mN m−1. Acetic acid was added to the water sub-phase to initiate the Schiff-base reaction. The reaction was left undisturbed at room temperature. The surface pressure area was recorded by compressing the Teflon barrier. Subsequently, the film was transferred to substrates via Langmuir–Blodgett transfer.48
Fig. 5. (a) Structure and LB synthesis of SL-2DP. Reproduced with permission from ref. 48. Copyright 2023 Wiley-VCH. (b) Synthesis of boronate-ester-linked 2D porphyrin-based COF films via the SMAIS method. Reproduced with permission from ref. 16. Copyright 2020 Wiley-VCH. (c) Schematic of the preparation of 2D COF films through a PSS-mediated on-water surface synthesis. Reproduced with permission from ref. 57. Copyright 2022 American Chemical Society.
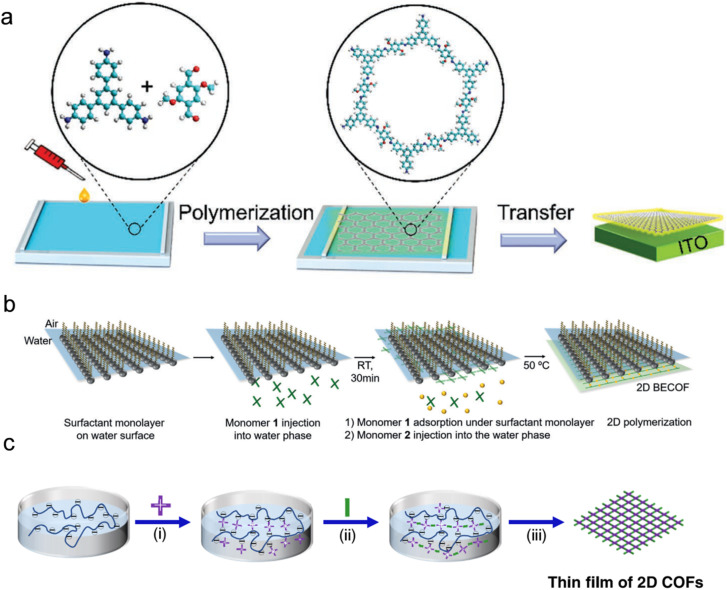
In an important breakthrough, in 2019, Feng and co-workers developed the surfactant monolayer-assisted interfacial synthesis (SMAIS) method to guide the preorganization and 2D polycondensation of rigid monomers on the surface of water. Generally, the SMAIS method includes three steps (Fig. 5b): (1) an organized surfactant monolayer is constructed by spreading a molecular surfactant on the water surface to guide the supramolecular arrangement. (2) An aqueous solution of monomer 1 with catalysts is injected to the water. Subsequently, the system is left undisturbed to allow the monomers to diffuse, be absorbed, and pre-organize under the surfactant monolayer. (3) The aqueous solution of monomer 2 with the catalyst is added to initiate 2D interfacial polymerization on the water surface. Importantly, the selection of surfactants was guided by their potential interactions with the monomer precursors, such as electrostatic interactions, hydrogen bonding, coordination bonds and strong covalent bonds. Common surfactants for SMAIS include sodium oleyl sulfate (SOS), stearic acid (SA), sodium dodecyl sulfate (SDS), sodium 4-dodecylbenzenesulfonate (SDBS), and cetyltrimethylammonium bromide (CTAB). In the past five years, Feng and co-workers have successfully synthesized various highly crystalline 2D films, including a 2D polyamide film,50 quasi-2D polyaniline film,51 2D porphyrin-based polyimide film,52 2D porphyrin-based polyboronate ester film,16 2D porphyrin-based polyimine film,53 2D poly(pyridinium salt) film,54 and vinylene-linked 2DPs film.55
Rather than a small-molecule surfactant monolayer, Zheng and co-workers used poly(acrylic acid) and poly(sodium 4-styrenesulfonate) (PSS) as polymeric surfactants for SMAIS.56
During the synthesis, negatively charged PSS was first spread over the air–water interface, which guided the assembly of the protonated monomers, polymerization and crystallization. For example, a wafer-sized thin film of COF-2,5-Ph was prepared by the Schiff base condensation of 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphyrin (TAPP) with 2,5-dihydroxy-terephthalaldehyde (2,5-Ph) (Fig. 5c). The thin films achieved an average single-crystalline domain size of around 3.57 ± 2.57 μm2.57 In May 2024, this group published a report in Nature detailing a sacrificial go-between guided interfacial synthesis to produce highly strong, tough and elastic 2D single-crystal COF films. Diethylenetriamine served as the go-between, and polyacrylic acid served as the polymeric water surface surfactant for the accumulation and assembly of protonated TAPP through static and hydrogen interactions. 18 h after adding aldehyde monomers to the water, a homogeneous film with large single-crystal domains was obtained, and could be transferred onto arbitrary substrates. The films were connected by an interwoven grain boundary, which endowed them with high strength, toughness and elasticity.58
3.4. Water-phase synthesis
Due to its green and low-cost characteristics, water has recently been considered as a promising reaction medium for synthesizing COFs.59–61 Common organic monomers have poor solubility in water, which limits the reactions between monomers. Thus, amphiphilic surfactants are used to stabilize the oil–water interface or form a micellar reaction system for synthesizing crystalline COFs. The condensation reaction can be confined on the surfactant-stabilized interface or inside the surfactant nano-compartments.18,44 In addition, a molecule/water interface was directly created for the synthesis of COFs in water-phase synthesis.
In 2020, Puigmartí-Luis and co-workers developed a biomimetic synthetic method for preparing sub-20 nm imine-based COF aqueous colloidal solutions at room temperature and ambient pressure.18 As shown in Fig. 6a, the authors employed a catanionic micellar system as reaction nano-compartments; a 97 : 3 mixture of the cationic surfactant CTAB and anionic surfactant sodium dodecyl sulfate (SDS) was used. In this system, the micellar medium enables the insoluble building blocks (TAPB and BTCA) to be soluble in water at room temperature. The two produced homogeneous solutions of the reactants and acetic acid were mixed to yield the crystalline TAPB–BTCA COF colloids. The reaction mixture was clear and homogeneous with no apparent precipitation (Fig. 6b). Cryo-TEM of the reaction mixture after 24 h showed two different objects with diameters of 5 ± 1 nm and 16 ± 1 nm (Fig. 6c). In addition, Wu and co-workers demonstrated a surfactant-free synthesis of C N– linked COF nanospheres in water at room temperature.62 A small amount of methyl acetate was adopted as the solvent for TAPB and 2,5-divinylterephthalaldehyde. Then, an aqueous solution of acetic acid was slowly added to the methyl acetate/monomer mixture at a rate of one drop per second. The water-phase system was left undisturbed for 72 h to produce the crystalline COFs. In this surfactant-free synthesis, the formative methyl acetate/water interface, the slow diffusion of the co-solvent and the rapid Schiff base reaction of the monomers together resulted in the nanosphere morphology.
Fig. 6. (a) The water-phase synthesis of colloidal TAPB–BTCA COF. (b) Photograph of the transparent reaction solution. (c) Cryo-TEM image of TAPB–BTCA COF colloid. Reproduced with permission from ref. 18. Copyright 2020 American Chemical Society.
Recently, Xu and co-workers reported a facile, green approach for the synthesis of imine-linked COFs in an aqueous solution at room temperature.63 The authors achieved the pre-activation of aldehyde monomers using acetic acid to enhance their reactivity in aqueous solutions. As demonstrated by the authors, the aldehyde monomers are not soluble in water, while the 1,4-diaminebenzene (DB) and acetic acid are soluble in water. The insoluble aldehyde monomers reacted with DB along the created molecule/water interface. In this report, 16 COFs with different molecular structures were successfully synthesized.
3.5. Electrosynthesis
Electrochemical technologies, which have the advantages of environmentally friendliness, high efficiency and electric controllability, have been used to develop high-quality organic films.64 Methods for the electrosynthesis of COFs are divided into two categories: (1) electrophoretic deposition (EPD), which focuses on the directional migration and assembly of charged COF nanoparticles on the electrodes by applying an electric field to their dispersions. (2) Electrosynthesis, in which charged monomers around the electrode surface react directly to form films under an electric field.
In 2019, Medina and co-workers reported EPD techniques for preparing imine- or borate-ester-linked COF films from their COF suspensions (Fig. 7a).65 Briefly, COF nanoparticle suspensions were first prepared. Subsequently, electric fields ranging from 100 to 900 V cm−1 were applied between two electrodes. After 2 min of EPD, 25 cm2 films were obtained on the electrode surfaces. Wang and co-workers developed a novel two-cell EPD design (Fig. 7b), in which ionic COF nanosheets were assembled into a membrane in the middle of the electric field instead of at the surface of the electrodes.66 This strategy avoided typical problems including bubbles and acidic/alkaline micro-environments in the near-electrode region. As a result, ultrathin and homogenous COF membranes could be prepared within several minutes.
Fig. 7. (a) Schematic of the EPD setup. Reproduced with permission from ref. 65. Copyright 2019 American Chemical Society. (b) Scheme of the fabrication of COF membranes on an AAO substrate via EPD in an H-shaped cell. Reproduced with permission from ref. 66. Copyright 2023 Wiley-VCH. (c) Electrochemical interfacial polymerization of TpPa film on a PAN substrate. Reproduced with permission from ref. 68. Copyright 2023 Wiley-VCH. (d) Electrocleavage synthesis of COF films. Reproduced with permission from ref. 17. Copyright 2019 American Chemical Society.
Wang and co-workers reported a bottom-up electrosynthesis approach to obtain an ionic COF membrane (TpEB).67 In the system, TpEB films were prepared on ITO under an electric field of 50 V cm−1. A brown covering appeared after 3 h of electrosynthesis on the ITO surface. The electrochemical process showed that cationic TpEB nuclei were rapidly formed after mixing of the monomer solutions and migrated to the cathode under the electric field. Subsequently, the dynamic Schiff-base reaction impelled the crystallization of the deposited TpEB nuclei, producing a continuous and smooth film. Very recently, Jiang and co-workers fabricated COF films via an electrochemical interfacial polymerization strategy with self-healing and self-inhibiting effects.68N-Octanoic acid and methanol were chosen as an acid catalyst and reaction solvent (Fig. 7c). The protonated amine monomers and aldehyde monomer migrated to the cathode. Subsequently, through the electrochemical deprotonation reaction and electric field migration of the monomers, the interfacial polymerization was synergistically intensified and the crystallization process was strongly accelerated. The authors demonstrated two inherent effects in the electrochemical process: a self-healing effect for a defect-free morphology and a self-inhibiting effect for an ultrathin film. Based on the above electrochemical results, Qiu and co-workers synthesized electrochromic TPDA-DHBD via the Schiff base reaction.69 The TPDA-DHBD films with redox nature exhibit stable polymorphic colour variations under different applied voltages. Interestingly, Inagi and co-workers reported an in situ produced proton source as a Brønsted acid catalyst for the electrosynthesis of C N bonds, in which the proton sources were produced by the electrochemical oxidation of 1,2-diphenylhydrazine (DPH).70 The electrogenerated acid from DPH promoted the condensation reaction of amine and aldehyde monomers. The obtained COF films with controlled film thickness showed high crystallinity and porosity. Finally, this synthetic method was also applicable for the synthesis of various imine-based COFs with different 2D or 3D structures. Ma and co-workers developed a combination of electrocleavage and the EPD strategy to prepare imine-linked COF films on electrodes at room temperature (Fig. 7d).17 Typically, the electrochemical synthesis was performed in a three-electrode electrochemical cell with 0.1 M LiClO4 in DMF as the electrolyte solution and 0.01 M p-toluenesulfonic acid (PTSA) as a proton source. The COF powder was first exfoliated to nanosheets on the cathodic electrode though electrochemical reduction and protonation. Therewith, the exfoliated nanosheets migrated to the anode and underwent anodic oxidation, producing the crystalline COF film. For clearer comparison of the effectiveness of the various approaches, the reaction conditions for electrosynthesis are summarized in Table 1.
Table 1. Key reaction conditions in the electrosynthesis of COFs.
| Sample | Method | Catalyst | Electrochemical cell | Applied potential | Supporting electrolyte | Reaction time | Ref. |
|---|---|---|---|---|---|---|---|
| BDT-ETTA COF | EPD | No catalysts | Single-cell system | 100 to 900 V cm−1 | Ethyl acetate | 2 min | 65 |
| TpPa-SO3H | EPD | No catalysts | Two-cell system | 20 V cm−1 | Methanol | 2–10 min | 66 |
| TpEB | Electrosynthesis | Acetic acid | Single-cell system | 50 V cm−1 | Ethanol | 3 h | 67 |
| TpPa | Electrosynthesis | n-Octanoic acid | Single-cell system | −7 V | Methanol | 4 h | 68 |
| TPDA-DHBD | Electrosynthesis | 1,2-Diphenylhydrazine | Single-cell system | CV: −0.5 V to 0.5 V vs. Ag/Ag+ | 0.1 M Bu4NPF6 in nitromethane | 20 cycles | 70 |
| TPB-DMTP-COF | Electrocleavage and EPD | 0.01 M PTSA | Single-cell system | CV: 0 V to −2 V vs. Ag/Ag+ | 0.1 M LiClO4 in N,N-dimethylformamide | 10 cycles | 17 |
3.6. Sonochemical synthesis
Sonochemical synthesis is an economical and low-power-consumption method.71,72 The ultrasound synthesis of COFs is driven by acoustic cavitation, in which the formation, growth, and implosive collapse of bubbles occur as ultrasound waves cross through a liquid medium. Ultrasound has also been used to assist solvothermal reactions for controlling crystallization and accelerating gel formation.73
In 2012, Ahn and co-workers first reported a sonochemical method for the synthesis of COFs.74 The crystallization rate in the sonochemical synthesis was accelerated due to the formation and collapse of bubbles in solution, which produces exceedingly high local temperatures and pressures (>1000 bar), resulting in fast heating and cooling rates. COF-1 and COF-5 were synthesized via sonochemical synthesis using a 20 kHz, 500 W sonicator for 1 h. The prepared COF-1 exhibited a BET surface area of 719 m2 g−1, which was comparable to the reported 711 m2 g−1via solvothermal synthesis. The BET surface area of COF-5 was 2122 m2 g−1, which was significantly higher than the reported 1590 m2 g−1 for solvothermally prepared COF-5. Cooper and co-workers used a sonochemical synthesis to prepare nine imine-linked COFs in an acidic aqueous medium using a 20 kHz, 550 W sonicator, as shown in Fig. 8.75 The COFs were successfully synthesized in just 60 min. Their crystallinity and porosity were comparable to or better than those of their counterparts obtained via solvothermal routes. Taking advantage of these simple and efficient methods, the same groups screened 60 crystalline COFs via sonochemical synthesis for photocatalytic hydrogen peroxide production using water and oxygen.76 Simao and co-workers demonstrated that the morphology of the COF films was related to the rate of bubble formation and the maximum bubble size during the ultrasound process.77
Fig. 8. Sonochemical apparatus and conditions, and COFs synthesized via sonochemical synthesis. Adapted with permission from ref. 73. Copyright 2022 Springer Nature.
3.7. Single-solution phase synthesis
Single-solution phase synthesis involves monomers that are highly soluble in solvents and can polymerize to a COF at room temperature. Due to the lack of high energy and 2D growth templates, the crystallinity is usually low. To date, all examples prepared by room-temperature homogeneous synthesis are C N linked COFs without air sensitivity.
In 2021, Wang and co-workers reported meta-structured assemblies of 2D polymers in single-phase solution (dioxane/mesitylene; v/v = 2/3). TAPA-TFPA was synthesized under the catalysis of Sc(OTf)3 in the presence of both aniline and benzaldehyde as competitors. Although the reaction finished at room temperature within 1 day, flower-shaped COFs showed high crystallinity.78 Recently, Jiang and co-workers reported the single solution-phase synthesis of charged COF-NSs with an ultrahigh volume yield of 18.7 mg mL−1 at room temperature (Fig. 9a). The negatively charged 2,5-diaminobenzenesulfonic acid and 1,3,5-triformylphloroglucinol were selected for polymerization. Dimethyl sulfoxide was selected as the organic solvent. After 24 h at room temperature, the obtained COF showed a spongy solid morphology with aggregated sheets several tens of micrometers in width (Fig. 9b). The TEM image exhibited high-order fringes (Fig. 9c). The mechanism of COF-NS growth indicated that charge-induced electrostatic repulsion forces enable in-plane anisotropic secondary growth from initial discrete and disordered polymers into crystalline COF-NSs.79
Fig. 9. (a) Room-temperature single solution-phase synthesis process. (b) SEM image. (c) HR-TEM image. Adapted with permission from ref. 79. Copyright 2023 Wiley-VCH.
Li and co-workers synthesized the first example of Au-MOFs (JNM-Au-n, n = 1, 2, 3, 4) via the room-temperature Schiff base reaction between cyclic trinuclear gold(i) complexes and aldehyde monomers in mesitylene.80 Astonishingly, the rapid single-phase synthesis can achieve scalable production (up to 1 g) in 15 min. Very recently, our groups reported the room-temperature single-phase synthesis of semiconducting COFs in DMF.25 Three COFs were rapidly prepared in 18 h based on the Schiff base reaction between C3v monomers with tunable planarity and metallized monomers at room temperature. Notably, the room-temperature single-phase synthesis conveniently allowed unprecedented insights into the crystallization mechanism of COFs. A competitive relationship existed between the growth of disordered structures and crystal nuclei. It was found that a twisted structure caused the rapid formation of amorphous structures and a slow dynamic crystallization rate, while highly planar monomers boosted the formation and the rapid growth of crystal nuclei. Very recently, Dong and co-workers reported the first example of β-ketoamine-linked COFs via room temperature Mannich reaction, in which a mixture of methanol (2.6 mL) and acetonitrile (1.3 mL) was used as the reaction solvent and CeCl3 served as the catalyst.81 After 7 days of the condensation polymerization of 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)tribenzaldehyde, acetophenone, and 4,4′-diaminodiphenyl, red TAD-COF solids were successfully prepared in 75.1% yield.
3.8. Mechanochemical synthesis
Mechanochemical reactions are induced by the direct absorption of mechanical energy. In mechanochemical synthesis, the chemical bonds are formed during the grinding of solid reactants in a mortar or ball mill.
Banerjee and co-workers reported the first solvent-free room-temperature mechanochemical synthesis of three β-ketoenamine-linked COFs (TpPa-1, TpPa-2, and TpBD) using a mortar and pestle (Fig. 10a).82 The grinding reaction time was only 40 min. XRD analysis of the β-ketoenamine-linked COFs showed high crystallinity. Unsatisfactorily, three COFs exhibited small BET surface areas of 61 m2 g−1 for TpPa-1, 56 m2 g−1 for TpPa-2, and 35 m2 g−1 for TpBD, which were lower than those of the corresponding products obtained via solvothermal synthesis.
Fig. 10. (a) Scheme of the mechanochemical synthesis of TpPa-1, TpPa-2, and TpBD through simple grinding using a mortar and pestle. Reproduced with permission from ref. 82. Copyright 2013 American Chemical Society. (b) Photographs of the reaction mixture for the synthesis of COF-1 before and after ball-milling. Adapted with permission from ref. 26. Copyright 2024 Wiley-VCH.
Furthermore, this group investigated liquid-assisted grinding (LAG) via adding a few drops of organic solvent and aqueous acid catalyst to the monomer mixture.83 A new crystalline hydrazone-linked COF (TpTh) and reported materials such as DhaTph and LZU-1 were successfully prepared by applying the LAG approach. Similarly, Sukumaran and co-workers synthesized 1 kg of Tp-Azo via the LAG approach with a planetary mixer.84 Cheetham and coworkers reported a cocrystal precursor template strategy via liquid-assisted mechanochemical synthesis in a ball mill.27 Benefitting from the cocrystal precursor, interlayer hydrogen bonding between the amine and PTSA created a cocrystal template for the condensation reaction with aldehydes, and then the aldehydes reacted via confined polymerization from the cocrystal synthons to COFs. Very recently, Perepichka and co-workers reported the first mechanochemical synthesis of boroxine-linked COF-1 (ref. 26) and the first example of a mechanochemically prepared 3D COF (COF-102). Using trimethyl-boroxine as a dehydrating additive, the hydrolytic sensitivity of the boroxine-based COFs was overcome. The resulting COFs showed high crystallinity and porosity. As shown in Fig. 10b, COF-1 can be synthesized rapidly via ball-milling. By using resonant acoustic mixing (RAM), which enabled direct input of mechanical energy to the samples, COF-1 was readily scaled up to 10 grams.
3.9. High-energy ionizing radiation synthesis
Due to their ionization of the entire reaction system, high-energy ionizing radiation sources such as γ rays and electron beams have been utilized to produce extremely reactive species, which enable the reagents to directly gain energy to bypass the activation barrier of the reaction and hasten the reaction process.
In 2020, Wang and co-workers reported the radiation-induced synthesis of 2D imine COFs via a high energy electron beam (Fig. 11a).85 Typically, COF precursors and an aqueous acetic acid catalyst were sealed under a nitrogen atmosphere. The system was irradiated by the electron beam for 160 s with a cumulative absorbed dose of 100 kGy. As the electron beam dose was increased, it was found that lower doses produced poor crystallinity, while higher doses resulted structural degradation of the COFs. In addition, first-principles calculations demonstrated that the free radicals (e.g. H˙, ˙OH, and R-NH˙) formed under irradiation can activate the intermediate and lower the reaction barrier. The same groups synthesized polymer-grafted COFs in situ via a simple 60Co γ-ray irradiated one-pot reaction under ambient conditions, in which the Schiff base reaction and free radical polymerization can occur simultaneously.86 In addition, Chou and co-workers adopted gamma irradiation to synthesize imine-linked and imide-linked COFs at room temperature under open-air conditions within 1 h (Fig. 11b).87 As shown in Fig. 11c, with increased γ-ray intensity, the resulting COF showed increased crystallinity. In addition, compared with their counterparts synthesized via the solvothermal method, the COFs obtained via gamma irradiation showed improved crystallinity, surface area, and thermal stability.
Fig. 11. (a) Experimental setup of the electron beam accelerator and the synthesized crystalline COFs. Reproduced with permission from ref. 85. Copyright 2020 American Chemical Society. (b) Illustration of the gamma irradiation unit. (c) The synthetic set-up. (d) XRD patterns of products obtained under different γ-ray intensities. Adapted with permission from ref. 87. Copyright 2024 Wiley-VCH.
3.10. Photochemical synthesis
Photochemical synthesis involves the fact that under the illumination of light, organic molecules or groups can be activated to form new linkages compared with their counterparts in the ground state.88 Among the photochemical reactions, in terms of energy- and resource-saving requirements, “window ledge” reactions that can be directly activated by natural sunlight are important candidate reactions to prepare COFs.89 In addition, some highly-efficient photocatalytic reactions catalyzed under artificial light sources can also be used to synthesize COFs.90 The light intensity and light wavelength depend on the selected reaction type.
In 2022, Dong and co-workers first reported the sunlight-driven photocatalytic synthesis of a benzoxazole-linked COF (LZU-191) under ambient conditions (Fig. 12a).89 The photocatalytic cyclization reaction of 2,5-diamino-1,4-benzenediol dihydrochloride and 1,3,5-tris(4-formylphenyl)triazine in oxygen was conducted in dimethylacetamide using N-methyl-2-pyrrolidone as a base and TBA-eosin Y as a visible-light photocatalyst. Benzoxazole-linked LZU-191 was obtained as a deep yellow solid under natural sunlight irradiation for 48 h, exhibited high crystallinity with an intense (100) plane peak at 2.92° (Fig. 12b), and could effectively drive the visible-light-catalytic aerobic oxidation of sulfides to sulfoxides. Recently, this group also reported a photoredox-catalyzed multicomponent Petasis reaction for the synthesis of COFs under ambient conditions, in which aromatic aldehydes, amines, and potassium cyclo-hexyltrifluoroborate in the presence of [Ir(dtbbpy)(ppy)2]PF6 produced crystalline COFs at room temperature.90 Upon visible-light irradiation for 72 h, a series of COFs, including Cy-N3-COF, Cy-LZU-1, Cy-COF-42, and TPB-DMTP-COF, were successfully synthesized with excellent crystallinity and stability. In addition, photochemically induced cycloaddition reactions including [4 + 4]-cycloadditions of anthracenes and [2 + 2]-cycloadditions of olefins can be used to obtain COFs that show smart response behavior.91–94 Recently, Lim and coworkers presented a novel approach for synthesizing 2D COF thin films by combining photochemistry and a liquid flow system.95 The photochemical approach can offer spatially controllable energy sources for patternable COF films. Finally, an ultrasmooth patterned high-crystalline 2D COF film on hexagonal boron nitride was successfully fabricated.
Fig. 12. (a) Photochemical synthesis of LZU-191 under natural sunlight. (b) PXRD patterns of LZU-191; top insets: crystal structure and TEM image of LZU-191. Reproduced with permission from ref. 89. Copyright 2022 American Chemical Society.
Novel room-temperature synthetic strategies have been classified based on their use of green solvents, confined interfaces, assisted energy sources and crystallization factors (Table 2). Despite the obvious progress that has been made in the room-temperature synthesis of COFs, there is still a long way to go before its industrialization.
Table 2. Main parameters for the room-temperature synthesis of representative COFs.
| Sample | Method | Room-temperature conditions | Yield | BET surface area | Crystallinity | State | Ref. |
|---|---|---|---|---|---|---|---|
| COF-DT | Solid–liquid interfacial synthesis | 48 h | Not available | Not available | High crystallinity | COF film | 37 |
| Tp-Bpy | Liquid–liquid interfacial synthesis | 72 h | 23% | 1151 m2 g−1 | High crystallinity | COF film | 31 |
| 2DP | LB method | 12 h | Not available | Not available | High crystallinity | COF film | 48 |
| 2D COF-1 film | On-water surface synthesis | 18 h | Not available | Not available | High crystallinity | COF film | 58 |
| TAPB–BTCA COF | Water-phase synthesis | 72 h | 88% | 687 m2 g−1 | High crystallinity | COF colloid | 18 |
| TpEB | Electrosynthesis | 3 h | Not available | 239.3 m2 g−1 | High crystallinity | COF film | 67 |
| COF-1; COF-5 | Sonochemical synthesis | 20 kHz, 500 W sonicator, 1 h | 100% (COF-1); 50% (COF-5) | 719 m2 g−1; 2122 m2 g−1 | High crystallinity; high crystallinity | COF powder | 61 |
| JNM-Au-1 | Single-solution phase synthesis | 15 min | 99% | 351.01 m2 g−1 | High crystallinity | COF powder | 74 |
| TpPa-1 | Mechanochemical synthesis | Mortar and pestle, 40 min | 90% | 61 m2 g−1 | High crystallinity | COF powder | 82 |
| EB-COF-1 | High-energy ionizing radiation synthesis | Electron beam irradiation, 160 s | 92% | 738 m2 g−1 | High crystallinity | COF powder | 85 |
| LZU-191 | Photochemical synthesis | Natural sunlight, 48 h | 73% (1.21 g) | 1314 m2 g−1 | High crystallinity | COF powder | 89 |
4. Conclusions and perspectives
In this review, we have presented and discussed advanced characterization technologies and the emerging room-temperature synthetic techniques for the preparation of COF powders or COF films. A variety of synthetic methods have facilitated the rapid and green synthesis of COFs. Compared with high-temperature solvothermal synthesis, room-temperature synthesis is more eco-friendly and has lower energy consumption. The development of room-temperature synthesis will promote the large-scale and green preparation of COFs.
(1) Due to the rapid synthesis and non-oriented growth, most synthesized COFs are still polycrystalline. Some C N-linked COF single crystals have been synthesized. However, the development of a variety of COF single crystals is highly desirable but challenging. More efforts should be devoted to determining how to control the experimental conditions to promote single-crystal domains.
(2) The reactions that can be utilized for the room-temperature synthesis of COFs are limited. Thus, the development and design of more reactions for the room-temperature synthesis of COFs are highly desirable. Constructing more room temperature reactions would not only greatly broaden the diversity of COF structures but also enrich their chemistry and physics. Various reversible or irreversible reactions should be further explored for the room-temperature synthesis of COF powders and COF films.
(3) Controlling and revealing the framework growth process are interesting and very important for highly efficient room temperature synthesis. The crystallization mechanism will vary depending on the selected type of monomers, catalyst, reaction system, reaction type, the solubility of the monomers, etc. Thus, monitoring the crystallization process in detail via advanced in situ characterization technologies will drive the rapid development and application of COFs.
(4) Compared with those achieved via solvothermal synthesis, the diversity of COF structures and the topological design of COFs via room-temperature synthesis are still lacking. Thus, focusing on the design of chemical structures and topological structures is clearly important for exploring potential applications.
(5) In room-temperature synthesis, there are two challenges: (1) a longer reaction time may be needed due to the slower reaction rate; (2) a low reaction temperature may not meet the activation energy requirements for novel condensation reactions. Thus, key assistance technologies must be developed to accelerate room-temperature synthesis. In future, exploring novel approaches to highly crystalline COFs using eco-friendly solvents and low-energy assistance methods will be more attractive. Faster and more-efficient synthesis are becoming the research focus in the COF field.
Data availability
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
D. Wu designed the structure of the review and wrote the original draft. N. Gu, J. Yao, Y. Cao, L. Wang, I. Shakir collected the papers for this review topic. Y. Xu and Y. Sun supervised the review and revised the manuscript. Finally, the manuscript was revised by all authors.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge the financial supports from National Natural Science Foundation of China (52473221, 22022510), Shanxi Provincial Natural Science Foundation (202403021212051, TZLH20230818009, 202204021301049, 202304021301030 YDZJSX20231A060, 202403021212121), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2024L166). I. S. acknowledges a research grant funded by the Research, Development, and Innovation Authority (RDIA) – Kingdom of Saudi Arabia – with grant number (12615-iu-2023-IU-R-2-1-EI-).
Notes and references
- Geng K. He T. Liu R. Dalapati S. Tan K. T. Li Z. Tao S. Gong Y. Jiang Q. Jiang D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020;120:8814–8933. doi: 10.1021/acs.chemrev.9b00550. [DOI] [PubMed] [Google Scholar]
- Belowich M. E. Stoddart J. F. Dynamic imine chemistry. Chem. Soc. Rev. 2012;41:2003–2024. doi: 10.1039/C2CS15305J. [DOI] [PubMed] [Google Scholar]
- Xu S. Richter M. Feng X. Vinylene-Linked Two-Dimensional Covalent Organic Frameworks: Synthesis and Functions. Acc. Mater. Res. 2021;2:252–265. doi: 10.1021/accountsmr.1c00017. [DOI] [Google Scholar]
- Liu Y. Fu S. Pastoetter D. L. Khan A. H. Zhang Y. Dianat A. Xu S. Liao Z. Richter M. Yu M. Položij M. Brunner E. Cuniberti G. Heine T. Bonn M. Wang H. I. Feng X. Vinylene-Linked 2D Conjugated Covalent Organic Frameworks by Wittig Reactions. Angew. Chem., Int. Ed. 2022;61:e202209762. doi: 10.1002/anie.202209762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F. Bi S. Sun Z. Wu D. Zhang F. 2,4,6-Trimethylpyridine-Derived Vinylene-Linked Covalent Organic Frameworks for Confined Catalytic Esterification. Angew. Chem., Int. Ed. 2022;61:e202210447. doi: 10.1002/anie.202210447. [DOI] [PubMed] [Google Scholar]
- Bi S. Meng F. Wu D. Zhang F. Synthesis of Vinylene-Linked Covalent Organic Frameworks by Monomer Self-Catalyzed Activation of Knoevenagel Condensation. J. Am. Chem. Soc. 2022;144:3653–3659. doi: 10.1021/jacs.1c12902. [DOI] [PubMed] [Google Scholar]
- Liu L. Gong Y. Tong Y. Tian H. Wang X. Hu Y. Huang S. Huang W. Sharma S. Cui J. Jin Y. Gong W. Zhang W. Imidazole-Linked Fully Conjugated Covalent Organic Framework for High-Performance Sodium-Ion Battery. CCS Chem. 2024;6:1255–1263. doi: 10.31635/ccschem.024.202403938. [DOI] [Google Scholar]
- Arora N. Flores C. Senarathna Milinda C. Thompson Christina M. Smaldone Ronald A. Design, Synthesis, and Applications of Mesoporous Covalent Organic Frameworks. CCS Chem. 2023;6:57–68. doi: 10.31635/ccschem.023.202303261. [DOI] [Google Scholar]
- Zhang M. Lu M. Yang M.-Y. Liao J.-P. Liu Y.-F. Yan H.-J. Chang J.-N. Yu T.-Y. Li S.-L. Lan Y.-Q. Ultrafine Cu nanoclusters confined within covalent organic frameworks for efficient electroreduction of CO2 to CH4 by synergistic strategy. eScience. 2023;3:100116. doi: 10.1016/j.esci.2023.100116. [DOI] [Google Scholar]
- Liu Y. Fu S. Pastoetter D. L. Khan A. H. Zhang Y. Dianat A. Xu S. Liao Z. Richter M. Yu M. Položij M. Brunner E. Cuniberti G. Heine T. Bonn M. Wang H. I. Feng X. Vinylene-Linked 2D Conjugated Covalent Organic Frameworks by Wittig Reactions. Angew. Chem., Int. Ed. 2022;61:e202209762. doi: 10.1002/anie.202209762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou K. Meng F. Zhang Z. Li X. Li M. Jiao Y. Wang Z. Bai X. Zhang F. Pyridazine-Promoted Construction of Vinylene-Linked Covalent Organic Frameworks with Exceptional Capability of Stepwise Water Harvesting. Angew. Chem., Int. Ed. 2024;63:e202402446. doi: 10.1002/anie.202402446. [DOI] [PubMed] [Google Scholar]
- Feng B. Chen X. Yan P. Huang S. Lu C. Ji H. Zhu J. Yang Z. Cao K. Zhuang X. Isomeric Dual-Pore Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2023;145:26871–26882. doi: 10.1021/jacs.3c09559. [DOI] [PubMed] [Google Scholar]
- Fu G. Yang D. Xu S. Li S. Zhao Y. Yang H. Wu D. Petkov P. S. Lan Z.-A. Wang X. Zhang T. Construction of Thiadiazole-Bridged sp2-Carbon-Conjugated Covalent Organic Frameworks with Diminished Excitation Binding Energy Toward Superior Photocatalysis. J. Am. Chem. Soc. 2024;146:1318–1325. doi: 10.1021/jacs.3c08755. [DOI] [PubMed] [Google Scholar]
- Yuan S. Li X. Zhu J. Zhang G. Van Puyvelde P. Van der Bruggen B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019;48:2665–2681. doi: 10.1039/C8CS00919H. [DOI] [PubMed] [Google Scholar]
- Wang H. Zeng Z. Xu P. Li L. Zeng G. Xiao R. Tang Z. Huang D. Tang L. Lai C. Jiang D. Liu Y. Yi H. Qin L. Ye S. Ren X. Tang W. Recent progress in covalent organic framework thin films: fabrications, applications and perspectives. Chem. Soc. Rev. 2019;48:488–516. doi: 10.1039/C8CS00376A. [DOI] [PubMed] [Google Scholar]
- Park S. Liao Z. Ibarlucea B. Qi H. Lin H.-H. Becker D. Melidonie J. Zhang T. Sahabudeen H. Baraban L. Baek C.-K. Zheng Z. Zschech E. Fery A. Heine T. Kaiser U. Cuniberti G. Dong R. Feng X. Two-Dimensional Boronate Ester Covalent Organic Framework Thin Films with Large Single Crystalline Domains for a Neuromorphic Memory Device. Angew. Chem., Int. Ed. 2020;59:8218–8224. doi: 10.1002/anie.201916595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Xu C. Zhang W. Zhang Q. Zhao M. Zeng C. Jiang Q. Gu C. Ma Y. Electrocleavage Synthesis of Solution-Processed, Imine-Linked, and Crystalline Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2022;144:8961–8968. doi: 10.1021/jacs.1c13072. [DOI] [PubMed] [Google Scholar]
- Franco C. Rodríguez-San-Miguel D. Sorrenti A. Sevim S. Pons R. Platero-Prats A. E. Pavlovic M. Szilágyi I. Ruiz Gonzalez M. L. González-Calbet J. M. Bochicchio D. Pesce L. Pavan G. M. Imaz I. Cano-Sarabia M. Maspoch D. Pané S. de Mello A. J. Zamora F. Puigmartí-Luis J. Biomimetic Synthesis of Sub-20 nm Covalent Organic Frameworks in Water. J. Am. Chem. Soc. 2020;142:3540–3547. doi: 10.1021/jacs.9b12389. [DOI] [PubMed] [Google Scholar]
- Liu S. Wei L. Zeng T. Jiang W. Qiu Y. Yao X. Wang Q. Zhao Y. Zhang Y.-B. Single-Crystal Dynamic Covalent Organic Frameworks for Adaptive Guest Alignments. J. Am. Chem. Soc. 2024;146:34053–34063. doi: 10.1021/jacs.4c13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Reddy V. A. Ang M. C.-Y. Cui J. Khong D. T. Han Y. Loh S. I. Cheerlavancha R. Singh G. P. Rajani S. Strano M. S. Single-Crystal 2D Covalent Organic Frameworks for Plant Biotechnology. J. Am. Chem. Soc. 2023;145:12155–12163. doi: 10.1021/jacs.3c01783. [DOI] [PubMed] [Google Scholar]
- Han J. Feng J. Kang J. Chen J.-M. Du X.-Y. Ding S.-Y. Liang L. Wang W. Fast growth of single-crystal covalent organic frameworks for laboratory X-ray diffraction. Science. 2024;383:1014–1019. doi: 10.1126/science.adk8680. [DOI] [PubMed] [Google Scholar]
- Lv H. Zhu X. Mei J. Xia Y. Wang B. Recent progress of in situ characterization of LiNi1−x−yCoxMnyO2 cathodes for lithium metal batteries: a mini review. Nano Res. 2024;17:1384–1401. doi: 10.1007/s12274-023-5986-2. [DOI] [Google Scholar]
- Sasmal H. S. Halder A. Kunjattu H S. Dey K. Nadol A. Ajithkumar T. G. Ravindra Bedadur P. Banerjee R. Covalent Self-Assembly in Two Dimensions: Connecting Covalent Organic Framework Nanospheres into Crystalline and Porous Thin Films. J. Am. Chem. Soc. 2019;141:20371–20379. doi: 10.1021/jacs.9b10788. [DOI] [PubMed] [Google Scholar]
- Lin X.-M. Sun Y.-L. Chen Y.-X. Li S.-X. Li J.-F. Insights into electrocatalysis through in situ electrochemical surface-enhanced Raman spectroscopy. eScience. 2024:100352. doi: 10.1016/j.esci.2024.100352. https://dx.doi.org/10.1016/j.esci.2024.100352 [DOI] [Google Scholar]
- Wu D. Zhang Q. Yin S. Song C. Gu N. Wang D. Cai T. Zhang B. Room-Temperature Single-Phase Synthesis of Semiconducting Metal-Covalent Organic Frameworks With Microenvironment-Tuned Photocatalytic Efficiency. Small Methods. 2024:2401284. doi: 10.1002/smtd.202401284. https://dx.doi.org/10.1002/smtd.202401284 [DOI] [PubMed] [Google Scholar]
- Hamzehpoor E. Effaty F. Borchers T. H. Stein R. S. Wahrhaftig-Lewis A. Ottenwaelder X. Friščić T. Perepichka D. F. Mechanochemical Synthesis of Boroxine-linked Covalent Organic Frameworks. Angew. Chem., Int. Ed. 2024;63:e202404539. doi: 10.1002/anie.202404539. [DOI] [PubMed] [Google Scholar]
- Chen H. Feng D. Wei F. Guo F. Cheetham A. K. Hydrogen-bond-regulated mechanochemical synthesis of covalent organic frameworks: cocrystal precursor strategy for confined assembly. Angew. Chem., Int. Ed. 2024:e202415454. doi: 10.1002/anie.202415454. [DOI] [PubMed] [Google Scholar]
- Emmerling S. T. Germann L. S. Julien P. A. Moudrakovski I. Etter M. Friščić T. Dinnebier R. E. Lotsch B. V. In situ monitoring of mechanochemical covalent organic framework formation reveals templating effect of liquid additive. Chem. 2021;7:1639–1652. [Google Scholar]
- Wen Y. Ding S. Ma C. Jia P. Tu W. Guo Y. Guo S. Zhou W. Zhang X. Huang J. Zhang L. Shen T. Qiao Y. In situ TEM visualization of Ag catalysis in Li-O2 nanobatteries. Nano Res. 2023;16:6833–6839. doi: 10.1007/s12274-022-5359-z. [DOI] [Google Scholar]
- Wu D. Zhang Q. Wang X. Zhang B. Interface-confined synthesis of a nonplanar redox-active covalent organic framework film for synaptic memristors. Nanoscale. 2023;15:2726–2733. doi: 10.1039/D2NR06904K. [DOI] [PubMed] [Google Scholar]
- Dey K. Pal M. Rout K. C. Kunjattu H S. Das A. Mukherjee R. Kharul U. K. Banerjee R. Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2017;139:13083–13091. doi: 10.1021/jacs.7b06640. [DOI] [PubMed] [Google Scholar]
- Zheng Q. Ren A. Zagalskaya A. Mao H. Lee D. Yang C. Bustillo K. C. Wan L. F. Pham T. A. Reimer J. A. Zhang J. Liu Y. Zheng H. Multistep Growth Pathway of Covalent Organic Framework Onion Nanostructures. J. Am. Chem. Soc. 2024;146:34167–34175. doi: 10.1021/jacs.4c14196. [DOI] [PubMed] [Google Scholar]
- Yang C. Jiang K. Zheng Q. Li X. Mao H. Zhong W. Chen C. Sun B. Zheng H. Zhuang X. Reimer J. A. Liu Y. Zhang J. Chemically Stable Polyarylether-Based Metallophthalocyanine Frameworks with High Carrier Mobilities for Capacitive Energy Storage. J. Am. Chem. Soc. 2021;143:17701–17707. doi: 10.1021/jacs.1c08265. [DOI] [PubMed] [Google Scholar]
- He Y. Lin X. Fabricating compact covalent organic framework membranes with superior performance in dye separation. J. Membr. Sci. 2021;637:119667. doi: 10.1016/j.memsci.2021.119667. [DOI] [Google Scholar]
- Cao L. Liu X. Shinde D. B. Chen C. Chen I. C. Li Z. Zhou Z. Yang Z. Han Y. Lai Z. Oriented Two-Dimensional Covalent Organic Framework Membranes with High Ion Flux and Smart Gating Nanofluidic Transport. Angew. Chem., Int. Ed. 2022;61:e202113141. doi: 10.1002/anie.202113141. [DOI] [PubMed] [Google Scholar]
- Medina D. D. Rotter J. M. Hu Y. Dogru M. Werner V. Auras F. Markiewicz J. T. Knochel P. Bein T. Solid-Liquid Interfacial Engineered Large-Area Two-Dimensional Covalent Organic Framework Films. J. Am. Chem. Soc. 2015;137:1016–1019. doi: 10.1021/ja510895m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. Liu X. Shinde D. B. Chen C. Chen I. C. Li Z. Zhou Z. Yang Z. Han Y. Lai Z. Oriented Two-Dimensional Covalent Organic Framework Membranes with High Ion Flux and Smart Gating Nanofluidic Transport. Angew. Chem., Int. Ed. 2022;61:e202113141. doi: 10.1002/anie.202113141. [DOI] [PubMed] [Google Scholar]
- Hong J. Liu M. Liu Y. Shang S. Wang X. Du C. Gao W. Hua C. Xu H. You Z. Liu Y. Chen J. Solid-Liquid Interfacial Engineered Large-Area Two-Dimensional Covalent Organic Framework Films. Angew. Chem., Int. Ed. 2024;63:e202317876. doi: 10.1002/anie.202317876. [DOI] [PubMed] [Google Scholar]
- Kumar Mahato A. Bag S. Sasmal H. S. Dey K. Giri I. Linares-Moreau M. Carbonell C. Falcaro P. Gowd E. B. Vijayaraghavan R. K. Banerjee R. Crystallizing Sub 10 nm Covalent Organic Framework Thin Films via Interfacial-Residual Concomitance. J. Am. Chem. Soc. 2021;143:20916–20926. doi: 10.1021/jacs.1c09740. [DOI] [PubMed] [Google Scholar]
- Hota M. K. Chandra S. Lei Y. Xu X. Hedhili M. N. Emwas A.-H. Shekhah O. Eddaoudi M. Alshareef H. N. Electrochemical Thin-Film Transistors using Covalent Organic Framework Channel. Adv. Funct. Mater. 2022;32:2201120. doi: 10.1002/adfm.202201120. [DOI] [Google Scholar]
- Gao S. Li Z. Yang Y. Wang Z. Wang Y. Luo S. Yao K. Qiu J. Wang H. Cao L. Lai Z. Wang J. The Ionic Liquid-H2O Interface: A New Platform for the Synthesis of Highly Crystalline and Molecular Sieving Covalent Organic Framework Membranes. ACS Appl. Mater. Interfaces. 2021;13:36507–36516. doi: 10.1021/acsami.1c08789. [DOI] [PubMed] [Google Scholar]
- Yadav D. Kumar A. Kim J. Y. Park N.-J. Baeg J.-O. Interfacially synthesized 2D COF thin film photocatalyst: efficient photocatalyst for solar formic acid production from CO2 and fine chemical synthesis. J. Mater. Chem. A. 2021;9:9573–9580. doi: 10.1039/D1TA00802A. [DOI] [Google Scholar]
- Matsumoto M. Valentino L. Stiehl G. M. Balch H. B. Corcos A. R. Wang F. Ralph D. C. Mariñas B. J. Dichtel W. R. Lewis-Acid-Catalyzed Interfacial Polymerization of Covalent Organic Framework Films. Chem. 2018;4:308–317. [Google Scholar]
- Ma Y. Yu S. Li W. Chen D. Zheng Z. Mao L. Yang X. Wang W.-J. Liu P. Rapid yet Controlled Synthesis of 2D Covalent Organic Framework Nanocapsules as High-Performance Photocatalytic Carriers. Angew. Chem., Int. Ed. 2024:e202416980. doi: 10.1002/anie.202416980. [DOI] [PubMed] [Google Scholar]
- Zhou D. Tan X. Wu H. Tian L. Li M. Synthesis of C–C Bonded Two-Dimensional Conjugated Covalent Organic Framework Films by Suzuki Polymerization on a Liquid-Liquid Interface. Angew. Chem., Int. Ed. 2019;58:1376–1381. doi: 10.1002/anie.201811399. [DOI] [PubMed] [Google Scholar]
- Wu D. Che Q. He H. El-Khouly M. E. Huang S. Zhuang X. Zhang B. Chen Y. Room-Temperature Interfacial Synthesis of Vinylene-Bridged Two-Dimensional Covalent Organic Framework Thin Film for Nonvolatile Memory. ACS Mater. Lett. 2023;5:874–883. [Google Scholar]
- Li Y. Wang Y. Zou Y. Zhang X. Dong H. Hu W. Two-Dimensional Conjugated Polymer Synthesized by Interfacial Suzuki Reaction: Towards Electronic Device Applications. Angew. Chem., Int. Ed. 2020;59:9403–9407. doi: 10.1002/anie.202002644. [DOI] [PubMed] [Google Scholar]
- Liu L. Geng B. Ji W. Wu L. Lei S. Hu W. A Highly Crystalline Single Layer 2D Polymer for Low Variability and Excellent Scalability Molecular Memristors. Adv. Mater. 2023;35:2208377. doi: 10.1002/adma.202208377. [DOI] [PubMed] [Google Scholar]
- Liu L. Ji W. He W. Cheng Y. Hao R. Hao P. Dong H. Ding X. Lei S. Han B. Hu W. Rational Design of Fluorinated 2D Polymer Film Based on Donor-Accepter Architecture toward Multilevel Memory Device for Neuromorphic Computing. Adv. Mater. 2024;36:2405328. doi: 10.1002/adma.202405328. [DOI] [PubMed] [Google Scholar]
- Liu K. Qi H. Dong R. Shivhare R. Addicoat M. Zhang T. Sahabudeen H. Heine T. Mannsfeld S. Kaiser U. Zheng Z. Feng X. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nat. Chem. 2019;11:994–1000. doi: 10.1038/s41557-019-0327-5. [DOI] [PubMed] [Google Scholar]
- Zhang T. Qi H. Liao Z. Horev Y. D. Panes-Ruiz L. A. Petkov P. S. Zhang Z. Shivhare R. Zhang P. Liu K. Bezugly V. Liu S. Zheng Z. Mannsfeld S. Heine T. Cuniberti G. Haick H. Zschech E. Kaiser U. Dong R. Feng X. Engineering crystalline quasi-two-dimensional polyaniline thin film with enhanced electrical and chemiresistive sensing performances. Nat. Commun. 2019;10:4225. doi: 10.1038/s41467-019-11921-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasoon A. Yu X. Hambsch M. Bodesheim D. Liu K. Zacarias A. Nguyen N. N. Seki T. Dianat A. Croy A. Cuniberti G. Fontaine P. Nagata Y. Mannsfeld S. C. B. Dong R. Bonn M. Feng X. Site-selective chemical reactions by on-water surface sequential assembly. Nat. Commun. 2023;14:8313. doi: 10.1038/s41467-023-44129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahabudeen H. Qi H. Ballabio M. Položij M. Olthof S. Shivhare R. Jing Y. Park S. Liu K. Zhang T. Ma J. Rellinghaus B. Mannsfeld S. Heine T. Bonn M. Cánovas E. Zheng Z. Kaiser U. Dong R. Feng X. Highly Crystalline and Semiconducting Imine-Based Two-Dimensional Polymers Enabled by Interfacial Synthesis. Angew. Chem., Int. Ed. 2020;59:6028–6036. doi: 10.1002/anie.201915217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Zhang Z. Qi H. Ortega-Guerrero A. Wang L. Xu K. Wang M. Park S. Hennersdorf F. Dianat A. Croy A. Komber H. Cuniberti G. Weigand J. J. Kaiser U. Dong R. Feng X. On-water surface synthesis of charged two-dimensional polymer single crystals via the irreversible Katritzky reaction. Nat. Synth. 2022;1:69–76. doi: 10.1038/s44160-021-00001-4. [DOI] [Google Scholar]
- Yang Y. Sabaghi D. Liu C. Dianat A. Mücke D. Qi H. Liu Y. Hambsch M. Xu Z.-K. Yu M. Cuniberti G. Mannsfeld S. C. B. Kaiser U. Dong R. Wang Z. Feng X. On-Water Surface Synthesis of Vinylene-Linked Cationic Two-Dimensional Polymer Films as the Anion-Selective Electrode Coating. Angew. Chem., Int. Ed. 2024;63:e202316299. doi: 10.1002/anie.202316299. [DOI] [PubMed] [Google Scholar]
- Tan F. Han S. Peng D. Wang H. Yang J. Zhao P. Ye X. Dong X. Zheng Y. Zheng N. Gong L. Liang C. Frese N. Gölzhäuser A. Qi H. Chen S. Liu W. Zheng Z. Nanoporous and Highly Thermal Conductive Thin Film of Single-Crystal Covalent Organic Frameworks Ribbons. J. Am. Chem. Soc. 2021;143:3927–3933. doi: 10.1021/jacs.0c13458. [DOI] [PubMed] [Google Scholar]
- Ou Z. Liang B. Liang Z. Tan F. Dong X. Gong L. Zhao P. Wang H. Zou Y. Xia Y. Chen X. Liu W. Qi H. Kaiser U. Zheng Z. Oriented Growth of Thin Films of Covalent Organic Frameworks with Large Single-Crystalline Domains on the Water Surface. J. Am. Chem. Soc. 2022;144:3233–3241. doi: 10.1021/jacs.1c13195. [DOI] [PubMed] [Google Scholar]
- Yang Y. Liang B. Kreie J. Hambsch M. Liang Z. Wang C. Huang S. Dong X. Gong L. Liang C. Lou D. Zhou Z. Lu J. Yang Y. Zhuang X. Qi H. Kaiser U. Mannsfeld S. C. B. Liu W. Gölzhäuser A. Zheng Z. Elastic films of single-crystal two-dimensional covalent organic frameworks. Nature. 2024;630:878–883. doi: 10.1038/s41586-024-07505-x. [DOI] [PubMed] [Google Scholar]
- Wang H. Wu D. Chen K. Gu N. Chen Y. Zhang B. Self-Template Hydrothermal Synthesis of Vinylene-Linked Covalent Organic Framework Nanosheets Confined at the Molecule/Water Interface for an Organic Memristor. ACS Mater. Lett. 2024;6:3376–3383. doi: 10.1021/acsmaterialslett.4c01128. [DOI] [Google Scholar]
- Zhang Z. Xu Y. Hydrothermal Synthesis of Highly Crystalline Zwitterionic Vinylene-Linked Covalent Organic Frameworks with Exceptional Photocatalytic Properties. J. Am. Chem. Soc. 2023;145:25222–25232. doi: 10.1021/jacs.3c08220. [DOI] [PubMed] [Google Scholar]
- Kim T. Joo S. H. Gong J. Choi S. Min J. H. Kim Y. Lee G. Lee E. Park S. Kwak S. K. Lee H.-S. Kim B.-S. Geomimetic Hydrothermal Synthesis of Polyimide-Based Covalent Organic Frameworks. Angew. Chem., Int. Ed. 2022;61:e202113780. doi: 10.1002/anie.202113780. [DOI] [PubMed] [Google Scholar]
- He J. Luo B. Zhang H. Li Z. Zhu N. Lan F. Wu Y. Surfactant-free synthesis of covalent organic framework nanospheres in water at room temperature. J. Colloid Interface Sci. 2022;606:1333–1339. doi: 10.1016/j.jcis.2021.07.026. [DOI] [PubMed] [Google Scholar]
- Kong X. Wu Z. Strømme M. Xu C. Ambient Aqueous Synthesis of Imine-Linked Covalent Organic Frameworks (COFs) and Fabrication of Freestanding Cellulose Nanofiber@COF Nanopapers. J. Am. Chem. Soc. 2024;146:742–751. doi: 10.1021/jacs.3c10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.-f. Liu T.-F. Cao R. An Electrochromic Hydrogen-Bonded Organic Framework Film. Angew. Chem., Int. Ed. 2020;59:22392–22396. doi: 10.1002/anie.202006926. [DOI] [PubMed] [Google Scholar]
- Rotter J. M. Weinberger S. Kampmann J. Sick T. Shalom M. Bein T. Medina D. D. Covalent Organic Framework Films through Electrophoretic Deposition—Creating Efficient Morphologies for Catalysis. Chem. Mater. 2019;31:10008–10016. doi: 10.1021/acs.chemmater.9b02286. [DOI] [Google Scholar]
- Wang R. Zhou Y. Zhang Y. Xue J. Caro J. Wang H. Ultrathin Covalent Organic Framework Membranes Prepared by Rapid Electrophoretic Deposition. Adv. Mater. 2022;34:2204894. doi: 10.1002/adma.202204894. [DOI] [PubMed] [Google Scholar]
- Wang X. Yang J. Shi X. Zhang Z. Yin C. Wang Y. Electrosynthesis of Ionic Covalent Organic Frameworks for Charge-Selective Separation of Molecules. Small. 2022;18:2107108. doi: 10.1002/smll.202107108. [DOI] [PubMed] [Google Scholar]
- Wang M. Wang Y. Zhao J. Zou J. Liang X. Zhu Z. Zhu J. Wang H. Wang Y. Pan F. Jiang Z. Electrochemical Interfacial Polymerization toward Ultrathin COF Membranes for Brine Desalination. Angew. Chem., Int. Ed. 2023;62:e202219084. doi: 10.1002/anie.202219084. [DOI] [PubMed] [Google Scholar]
- Wang Y.-A. Wu Q. Wang X. Jiang M. Zhang R. Chen X.-J. Liang R.-P. Qiu J.-D. In Situ Electrochemical Interfacial Polymerization for Covalent Organic Frameworks with Tunable Electrochromism. Angew. Chem., Int. Ed. 2024;63:e202413071. doi: 10.1002/anie.202413071. [DOI] [PubMed] [Google Scholar]
- Shirokura T. Hirohata T. Sato K. Villani E. Sekiya K. Chien Y.-A. Kurioka T. Hifumi R. Hattori Y. Sone M. Tomita I. Inagi S. Site-Selective Synthesis and Concurrent Immobilization of Imine-Based Covalent Organic Frameworks on Electrodes Using an Electrogenerated Acid. Angew. Chem., Int. Ed. 2023;62:e202307343. doi: 10.1002/anie.202307343. [DOI] [PubMed] [Google Scholar]
- Wei X. Bi M. Lou Q. Di D. Liu B. Pei D. Fast and facile sonochemical fabrication of covalent organic frameworks in water for the adsorption of flavonoids: adsorption behaviors and mechanisms. Colloids Surf., A. 2024;702:134731. [Google Scholar]
- Duan K. Wang J. Zhang Y. Liu J. J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. Membr. Sci. 2019;572:588–595. [Google Scholar]
- Wang Q. Gao L. Wang P. Wang Y. Xu Y. Xu H. Wang X. Meng Z. Xi K. Preparation of sp2 carbon-bonded π-conjugated COF aerogels by ultrasound-assisted mild solvothermal reaction for multi-functional applications. Nanoscale. 2024;16:15298–15307. doi: 10.1039/D4NR02017K. [DOI] [PubMed] [Google Scholar]
- Yang S.-T. Kim J. Cho H.-Y. Kim S. Ahn W.-S. Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method. RSC Adv. 2012;2:10179–10181. [Google Scholar]
- Zhao W. Yan P. Yang H. Bahri M. James A. M. Chen H. Liu L. Li B. Pang Z. Clowes R. Browning N. D. Ward J. W. Wu Y. Cooper A. I. Using sound to synthesize covalent organic frameworks in water. Nat. Synth. 2022;1:87–95. [Google Scholar]
- Zhao W. Yan P. Li B. Bahri M. Liu L. Zhou X. Clowes R. Browning N. D. Wu Y. Ward J. W. Cooper A. I. Accelerated Synthesis and Discovery of Covalent Organic Framework Photocatalysts for Hydrogen Peroxide Production. J. Am. Chem. Soc. 2022;144:9902–9909. doi: 10.1021/jacs.2c02666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C. J. F. Freitas S. K. S. de Sousa I. G. P. P. Esteves P. M. Simao R. A. Solvent role on covalent organic framework thin film formation promoted by ultrasound. Colloids Surf., A. 2020;585:124086. doi: 10.1016/j.colsurfa.2019.124086. [DOI] [Google Scholar]
- Wang S. Yang Y. Zhang H. Zhang Z. Zhang C. Huang X. Kozawa D. Liu P. Li B.-G. Wang W.-J. Toward Covalent Organic Framework Metastructures. J. Am. Chem. Soc. 2021;143:5003–5010. doi: 10.1021/jacs.0c13090. [DOI] [PubMed] [Google Scholar]
- Huang T. Jiang H. Douglin J. C. Chen Y. Yin S. Zhang J. Deng X. Wu H. Yin Y. Dekel D. R. Guiver M. D. Jiang Z. Single Solution-Phase Synthesis of Charged Covalent Organic Framework Nanosheets with High Volume Yield. Angew. Chem., Int. Ed. 2023;62:e202209306. doi: 10.1002/anie.202209306. [DOI] [PubMed] [Google Scholar]
- Wei R.-J. Xie M. Xia R.-Q. Chen J. Hu H.-J. Ning G.-H. Li D. Gold(I)-Organic Frameworks as Catalysts for Carboxylation of Alkynes with CO2. J. Am. Chem. Soc. 2023;145:22720–22727. doi: 10.1021/jacs.3c08262. [DOI] [PubMed] [Google Scholar]
- Wang J.-C. Sun T. Zhang J. Chen Z. Du J.-Q. Kan J.-L. Dong Y.-B. Construction of covalent organic frameworks via the Mannich reaction at room temperature for light-driven oxidative hydroxylation of arylboronic acids. Chemi. Sci. 2024;15:18634–18639. doi: 10.1039/D4SC04358H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. P. Chandra S. Kandambeth S. Lukose B. Heine T. Banerjee R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013;135:5328–5331. doi: 10.1021/ja4017842. [DOI] [PubMed] [Google Scholar]
- Das G. Balaji Shinde D. Kandambeth S. Biswal B. P. Banerjee R. Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 2014;50:12615–12618. doi: 10.1039/C4CC03389B. [DOI] [PubMed] [Google Scholar]
- Asokan K. Patil M. K. Mukherjee S. P. Sukumaran S. B. Nandakumar T. Scalable Mechanochemical Synthesis of β-Ketoenamine-linked Covalent Organic Frameworks for Methane Storage. Chem.–Asian J. 2022;17:e202201012. doi: 10.1002/asia.202201012. [DOI] [PubMed] [Google Scholar]
- Zhang M. Chen J. Zhang S. Zhou X. He L. Sheridan M. V. Yuan M. Zhang M. Chen L. Dai X. Ma F. Wang J. Hu J. Wu G. Kong X. Zhou R. Albrecht-Schmitt T. E. Chai Z. Wang S. Electron Beam Irradiation as a General Approach for the Rapid Synthesis of Covalent Organic Frameworks under Ambient Conditions. J. Am. Chem. Soc. 2020;142:9169–9174. doi: 10.1021/jacs.0c03941. [DOI] [PubMed] [Google Scholar]
- Zhang M. Yuan M. Zhao X. Chen J. He L. Gao Q. Hu J. Wu G. Chai Z. Wang S. Radiation-induced one-pot synthesis of grafted covalent organic frameworks. Sci. China Chem. 2023;66:1781–1787. doi: 10.1007/s11426-022-1532-8. [DOI] [Google Scholar]
- Elewa A. M. Mekhemer I. M. A. El-Mahdy A. F. M. Sabbah A. Chen S.-Y. Ting L.-Y. Abdelnaser S. Chou H.-H. Room-Temperature Synthesis of Covalent Organic Frameworks using Gamma-Irradiation in Open-Air Conditions. Small. 2024;20:2311472. doi: 10.1002/smll.202311472. [DOI] [PubMed] [Google Scholar]
- Kim S. Park C. Lee M. Song I. Kim J. Lee M. Jung J. Kim Y. Lim H. Choi H. C. Rapid Photochemical Synthesis of Sea-Urchin-Shaped Hierarchical Porous COF-5 and Its Lithography-Free Patterned Growth. Adv. Funct. Mater. 2017;27:1700925. doi: 10.1002/adfm.201700925. [DOI] [Google Scholar]
- Wu C.-J. Li X.-Y. Li T.-R. Shao M.-Z. Niu L.-J. Lu X.-F. Kan J.-L. Geng Y. Dong Y.-B. Natural Sunlight Photocatalytic Synthesis of Benzoxazole-Bridged Covalent Organic Framework for Photocatalysis. J. Am. Chem. Soc. 2022;144:18750–18755. doi: 10.1021/jacs.2c07893. [DOI] [PubMed] [Google Scholar]
- Wang G.-B. Wang Y.-J. Kan J.-L. Xie K.-H. Xu H.-P. Zhao F. Wang M.-C. Geng Y. Dong Y.-B. Construction of Covalent Organic Frameworks via a Visible-Light-Activated Photocatalytic Multicomponent Reaction. J. Am. Chem. Soc. 2023;145:4951–4956. doi: 10.1021/jacs.2c13541. [DOI] [PubMed] [Google Scholar]
- Acharjya A. Pachfule P. Roeser J. Schmitt F.-J. Thomas A. Vinylene-Linked Covalent Organic Frameworks by Base-Catalyzed Aldol Condensation. Angew. Chem., Int. Ed. 2019;58:14865–14870. doi: 10.1002/anie.201905886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. Oppenheim J. J. Skorupskii G. Yang L. Dincă M. Reversible topochemical polymerization and depolymerization of a crystalline 3D porous organic polymer with C-C bond linkages. Chem. 2022;8:3215–3224. [Google Scholar]
- Huang N. Ding X. Kim J. Ihee H. Jiang D. A Photoresponsive Smart Covalent Organic Framework. Angew. Chem., Int. Ed. 2015;54:8704–8707. doi: 10.1002/anie.201503902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav T. Fang Y. Liu C.-H. Dadvand A. Hamzehpoor E. Patterson W. Jonderian A. Stein R. S. Perepichka D. F. Transformation between 2D and 3D Covalent Organic Frameworks via Reversible [2 + 2] Cycloaddition. J. Am. Chem. Soc. 2020;142:8862–8870. doi: 10.1021/jacs.0c01990. [DOI] [PubMed] [Google Scholar]
- Kim T. Oh J. Kim S. C. Ahn J.-G. Kim S. Kim Y. Y. Lim H. Photochemical and Patternable Synthesis of 2D Covalent Organic Framework Thin Film Using Dynamic Liquid/Solid Interface. Small Methods. 2024:2400063. doi: 10.1002/smtd.202400063. https://dx.doi.org/10.1002/smtd.202400063 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.