Abstract
The precise and unambiguous detection and quantification of internal RNA modifications represents a critical step for understanding their physiological functions. The methods of direct RNA sequencing are quickly developing allowing for the precise location of internal RNA marks. This detection is, however, not quantitative and still presents detection limits. One of the biggest remaining challenges in the field is still the detection and quantification of m6A, m6Am, inosine, and m1A modifications of adenosine. The second intriguing and timely question remaining to be addressed is the extent to which individual marks are coregulated or potentially can affect each other. Here, we present a methodological approach to detect and quantify several key mRNA modifications in human total RNA and in mRNA, which is difficult to purify away from contaminating tRNA. We show that the adenosine demethylase FTO primarily targets m6Am marks in noncoding RNAs in HEK293T cells. Surprisingly, we observe little effect of FTO or ALKBH5 depletion on the m6A mRNA levels. Interestingly, the upregulation of ALKBH5 is accompanied by an increase in inosine level in overall mRNA.
Keywords: adenosine methylation, inosine, RNA editing, ALKBH5, FTO, ADAR
INTRODUCTION
The ever-growing number of RNA modifications raises the question of how to comprehensively identify different types of these modifications within a single RNA template/sample. One of the challenges is the method of choice to detect and quantify a particular RNA modification. Many methods initially relied on precipitating RNAs with a modification-specific antibody. However, the specificities of antibodies raised to different RNA modifications are highly variable (Grozhik et al. 2019). Over the past 5 years, there has been significant progress in establishing direct and indirect sequencing-based methods for the detection of individual RNA modifications (Schaefer et al. 2009; Dominissini et al. 2012; Meyer et al. 2012; Schwartz et al. 2014a; Ke et al. 2015; Linder et al. 2015). These approaches are excellent for qualitative detection of modifications, but are not yet so useful for quantitative assessments of multiple modifications in a given sample. To address this challenge, we employed liquid chromatography–tandem mass spectrometry (LC-MS/MS), an approach that can accurately detect and quantify selected relevant modifications in a single sample.
A significant question arises regarding how the presence of one RNA modification affects the occurrence of another. As N6-methyladenosine (m6A) and inosine affect the same position on the adenosine and are thus mutually exclusive, it is reasonable to assume that if any RNA modification could affect the occurrence of another, it would be these two (for review, see Rengaraj et al. 2021). A few reports showed a negative or positive correlation between m6A and inosine, but the methods used were not quantitative (Xiang et al. 2018; Li et al. 2022). Our goal here was to globally analyze whether there were significant differences in the presence of m6A or inosine when their occurrence was perturbed.
The conversion of adenosine to inosine (A-to-I) in double-stranded (ds) RNA is referred to as RNA editing and is catalyzed by the adenosine deaminase acting on RNA (ADAR) enzymes (for review, see Sinigaglia et al. 2019). Inosine can base pair with cytosine and is recognized primarily as guanosine during translation (Licht et al. 2019), potentially altering the coding sequence if present in exons. There are two active enzymes in mammals; ADAR1 and ADAR2. ADAR1 is ubiquitously expressed and is responsible for “promiscuous” editing. This involves editing of duplex regions in transcripts formed by inverted repetitive elements, such as Alu elements, with an editing level of <1% (for review, see Eisenberg and Levanon 2018). Because of the high prevalence of repetitive elements in humans, there are millions of positions edited in the human transcriptome. The expression of ADAR2 in humans is high in the arteries, lungs, bladder, and brain. In the brain, it edits a specific site in the GRIA2 transcript to 100%, whereas other mRNA targets show lower percentage of editing (Seeburg et al. 1998). We primarily focus on ADAR1 instead of ADAR2 due to its wider tissue expression.
Unlike adenosine deamination, N6-methylation (m6A) does not change the codon meaning when it is present in an open reading frame. It is more prevalent than inosine or any other internal mRNA modification (for review, see Murakami and Jaffrey 2022). Depending on the location, m6A has different effects on mRNA biogenesis and function (Zhou et al. 2024). It has been linked to alternative splicing, mRNA export, translation, or mRNA stability (for review, see Murakami and Jaffrey 2022). This modification is enriched in 3′ untranslated regions (UTRs) of mRNAs and around stop codons (Dominissini et al. 2012; Ke et al. 2015), however, its abundance and roles in pre-mRNAs remain for the most part unknown. Two different methyltransferases (referred to as the “writers”) can deposit m6A in mammalian mRNAs, the methyltransferase-like protein 3 and 14 heterodimer (METTL3 and METTL14) and the methyltransferase-like protein 16 (METTL16). METTL3/14 associates with additional auxiliary factors and is understood to be the main m6A writer responsible for the majority of m6A in mRNAs (Liu et al. 2014; Schwartz et al. 2014b; Poh et al. 2022). METTL16 appears to have a more limited substrate repertoire. It primarily modifies Mat2a mRNA and U6 snRNA (Pendleton et al. 2017; Warda et al. 2017) and few other mRNAs (Yoshinaga et al. 2022; Sun et al. 2023; for review, see Mansfield 2024), but it also has m6A-independent function (Wang et al. 2023). In this study, we primarily address the effect of METTL3/14 on the overall m6A levels.
M6A in mRNAs can be demethylated by at least two demethylases, the so-called “erasers”; the fat mass and obesity-associated protein (FTO) and/or the AlkB homolog 5 RNA demethylase (ALKBH5) (Jia et al. 2011; Zheng et al. 2013). FTO displays a broader substrate specificity as it can also target m6A in snRNAs, N6, 2′-O-dimethyladenosine (m6Am) in mRNAs and snRNAs, and m1A in tRNAs (Wei et al. 2018; Mauer et al. 2019). The extent of the N6-methyl group removal by the “erasers” is still controversial (Darnell et al. 2018), motivating us to investigate the potential dynamics of m6A and m6Am modifications in detail.
Although LC-MS/MS is considered the “gold standard” for the identification of RNA modifications, there are certain caveats to this method that have first to be addressed. These mainly concern the purity of the mRNA samples (Richter et al. 2021) and the method used to calculate the abundance of modifications. Studies that focus on mRNA require thorough sample purification. However, even after several rounds of poly(A) enrichment, “contaminating” noncoding RNAs such as rRNA can still be detected (Legrand et al. 2017). To address this concern, we measure tRNA- and rRNA-specific or -enriched modifications in parallel with the mRNA sample being analyzed to document mRNA purity in individual samples. We present methods for quantifying individual marks that avoid issues with the high abundance of adenosine representation in poly(A) enriched samples. Additionally, we also provide strategies for distinguishing and quantifying 5′ cap-linked and internal modifications. Using these methods, we show that m6A and RNA editing are interlinked in certain human cell types and uncover a minor role of FTO and ALKBH5 on modulating total m6A levels in steady-state mRNAs.
RESULTS
Establishment of LC-MS/MS analysis of RNA modifications
For the RNA sample preparation and LC-MS/MS analysis, we followed published protocols with some modifications (Thuring et al. 2016) (see Materials and Methods for details). Isotope-labeled internal standards (ISs) were used to determine the amount of RNA modification. These standards were prepared from total RNA isolated from Saccharomyces cerevisiae or Escherichia coli cultivated in 13C-labeled media. As m6Am is not present in bacteria or yeast, it was chemically synthesized as described in Kellner et al. (2014).
We optimized the quantification of modification in both total and poly(A)-enriched RNA samples isolated from T-Rex FlpIN HEK293 cells (293T). To distinguish and quantify internal and cap-associated modifications, we performed mass spectrometry analyses of RNA samples prepared in parallel with two different nuclease treatments. Nuclease P1 (NP1) cleavage of total RNA results in the digestion of all internal nucleotides to mononucleotides, whereas the first nucleotide remains linked to the cap (Mauer et al. 2019). This enables the measurement of modifications arising specifically from internal positions, which we term as “internal” throughout the text (Fig. 1A). To measure both internal and m7G cap-linked modifications (termed “cap + internal”), we used the combination of NP1 and snake venom phosphodiesterase I (SVP), which hydrolyzes nucleotide polyphosphates (Fig. 1A; Supplemental Fig. S1A). Cap-linked modifications were identified through treatment with both SVP and NP1 of total RNAs, revealing the presence of m6Am and m227G, corresponding to trimethylated extended caps of snRNAs (Fig. 1B). The poly(A) RNA samples showed as the most prominent marks m7G, m6Am, and Am (Fig. 1C).
FIGURE 1.
Quantitative LC-MS/MS measurements of 5′ terminal and internal modifications in total and poly(A) RNA fraction. (A) Schematic diagram illustrating the strategy for the detection of cap + internal (+NP1 +SVP) and internal (+NP1 −SVP) m6Am levels. NP1 cleaves after each nucleotide, releasing 5′ monophosphorylated nucleotides (pN), but is unable to cleave the triphosphate bond between the RNA cap (m7G or m227G [TMG] for mRNA and snRNA, respectively) and the first nucleotide. SVP hydrolyses nucleotide polyphosphates and can therefore digest the triphosphate bond, releasing cap-linked m6Am. The green and red arrows indicate the cleavage sites of SVP and NP1, respectively. Cap-linked m6Am in green, internal m6Am in red. (B) Nucleoside modification content in the cap structure of total RNA in HEK293T cells (n = 3). (C) Nucleoside modification content in the cap structure of poly(A)RNA after two steps of poly(A) selection in HEK293T cells (n = 3). (D) Quantification of m62A and m5U ratios in total RNA and poly(A) RNA enriched by one or two steps of poly(A) RNA isolations. The levels of nucleoside modifications were normalized to the total molar amount of canonical nucleosides N (N = C + U + G + A). n = 10. Data are average ± SD. <LOQ = under the limit of quantification. (E) Measurements of m6A and m6Am. (F) Relative levels of m5U, m62A, and m227G, marks specific for tRNA, 18S rRNA, and mature sm-class of snRNAs, respectively. Relative levels of m7G (G), adenosine (H), and inosine (I). The levels of nucleoside modifications were normalized to the total molar amount of canonical nucleosides N (N = C + U + G + A). n = 3, data are mean ± SD, unpaired two-tailed t-test. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. Source data are available online for this figure.
From the outset, we presumed that achieving 100% purity of mRNA is not possible as it only constitutes ∼2% of cellular RNA. The presence of noncoding RNAs was thus determined by monitoring 5-methyluridine (m5U), which is common to tRNA and rRNA (Powell and Minczuk 2020), N6,N6-dimethyladenosine (m62A) levels as an 18S rRNA-specific marker (Kellner et al. 2014) and N2,N2,7-trimethylguanosine (m227G) cap as a mark for Pol II snRNAs and snoRNAs (Supplemental Table S1; Saponara and Enger 1969; Terns and Dahlberg 1994; Franke et al. 2008; Wurth et al. 2014; Buemi et al. 2022). After optimization of various reagents and protocols, we achieved the best results by performing two rounds of polyadenylated RNA selection using two different poly(A) purification kits (Fig. 1D) (see Materials and Methods for details). Two rounds of poly(A) selection typically resulted in significant enrichment of the abundant mRNA marks m6A and m6Am (Fig. 1E), and significant depletion of the tRNA, rRNA, and snRNA modifications m5U, m62A, and m227G, respectively (Fig. 1F). The levels of m7G, a hallmark of capped mRNAs that can also be found in other Pol II-derived ncRNAs, were reduced upon two rounds of poly(A) enrichment, showing depletion of ncRNAs (Fig. 1G). We used the levels of canonical nucleotides for normalizations because mRNA enrichment led to a ∼20% increase in total adenosine levels, and thus calculating the ratio of mRNA modifications (e.g., inosine or m6A) to adenosine would lead to underestimating values (Fig. 1H). Compared to m6A, inosine levels in mRNA were by ∼6 orders lower than in total RNA (Fig. 1I), which is consistent with the previous data of high m6A prevalence in mRNAs and high prevalence of inosine in tRNAs (Fig. 1I; Torres et al. 2015). In summary, these results demonstrated the effectiveness of our approach to selectively enrich polyadenylated RNAs and to sufficiently deplete noncoding RNAs to be able to determine specific mRNA modifications.
ADAR1 does not regulate m6A levels in HEK293 and 293T cells
Next, we aimed to address whether there is any cross-regulation between adenosine deamination and N6-adenosine methylation. To this end, we used HEK293 and 293T cells to determine the levels of both modifications in mRNA isolated from cells depleted of either ADAR1 or METTL3 (Fig. 2A,B; Supplemental Fig. S2A,B). As expected, HEK293 cells expressing shRNA targeting ADAR1 show a significant decrease in inosine, and METTL3 knockdown (KD) by siRNAs in 293T cells resulted in the reduction of m6A levels (Fig. 2C,D). ADAR1 depletion did not alter the levels of m6A nor other measured marks (Fig. 2C; Supplemental Fig. S2A,C). Similarly, KD of METTL3 did not result in any changes in inosine or other examined modifications (Fig. 2D; Supplemental Fig. S2B,D). We did not observe any contamination of the poly(A) RNA with ncRNAs that could potentially affect the m6A and inosine levels (Supplemental Fig. S2C,D).
FIGURE 2.
ADAR1 and METTL 3 do not show cross-regulation in HEK293 and 293T cells. (A) Immunoblot analysis of protein expression in HEK293 cells treated with control (scrambled) or shRNA specific for ADAR1 mRNA. The levels of proteins were detected with specific antibodies listed in Materials and Methods. (B) Immunoblot analysis of protein expression in 293T cells transfected with control (scrambled) or METTL3 targeting siRNAs (METTL3 KD). Proteins were detected with specific antibodies listed in Material and Methods. (C) Levels of inosine, m6A, and m6Am in poly(A) RNA after KD of ADAR1 in HEK293 cell line (n = 3–4). (D) Levels of inosine, m6A, and m6Am in poly(A) RNA after KD of METTL3 in the 293T cell line (n = 4). Data are mean ± SD, unpaired two-tailed t-test. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. The levels of nucleoside modifications were normalized to the total molar amount of canonical nucleosides N (N = C + U + G + A). Source data are available online for this figure.
The m6Am in ncRNAs is the primary target of FTO
To elucidate the dynamic potential of two common RNA modifications, m6A and m6Am, two knockout (KO) cell lines of ALKBH5 KO or FTO KO were generated in 293T cells (Fig. 3A). Both FTO and ALKBH5 were previously identified as m6A demethylases, but subsequent studies have shown that FTO exhibited a preference for m6Am (Mauer et al. 2017; Wei et al. 2018). We initially focused on the functions of the demethylases on total RNA. As anticipated, we observed a significant increase in m6Am levels in FTO KO cells, whereas the levels of m6Am remained unchanged in ALKBH5 KO cells (Fig. 3B). Levels of m6A and m6Am methyltransferases remained unaffected in both cell lines (Supplemental Fig. S3A). The observed increase in m6Am levels in FTO KO cells was evident only upon SVP treatment, indicating that cap-linked m6Am is a primary target of FTO (Fig. 3B). In contrast, internal m6Am levels remained unaffected in FTO KO cells (Fig. 3B). This is consistent with previously published data by Mauer et al. (2019) on the demethylation role of FTO on cap-linked m6Am in snRNAs. Importantly, episomal expression of FTO WT, but not catalytically inactive (HD mutant) or disease-associated (RQ mutant, Boissel et al. 2009) FTO, was able to restore m6Am levels back down to those observed in WT cells (Fig. 3C). The m6A levels in total RNA remained unaltered following the KO of either of the demethylases (Supplemental Fig. S3B).
FIGURE 3.
FTO primarily targets cap-associated m6Am in noncoding RNAs. (A) Immunoblot analysis of protein expression in 293T ALKBH5 KO and FTO KO cells. The levels of proteins were detected with specific antibodies listed in Materials and Methods. (B) LC-MS/MS measurements and quantification of relative levels of internal (−SVP) and cap + internal m6Am in total RNA isolated from WT, FTO KO, and ALKBH5 KO 293T cells. One microgram of total RNA was digested with NP1 and with or without SVP, to release or not the cap-linked nucleotide, respectively. In both cases, dephosphorylation with shrimp alkaline phosphatase (SAP) was performed prior to LC-MS/MS analysis (n = 3). (C) Relative levels of m6Am in total RNA digested with NP1 and SVP, isolated from WT, FTO KO, and FTO KO cells with stably integrated forms of FTO WT (wild type), RQ (disease-linked mutant), and HD (catalytically inactive) (n = 3). (D) LC-MS/MS measurements of m6A, m6Am, inosine, and m7G in poly(A) RNA isolated from WT and FTO KO cells (n = 8–9). Data are mean ± SD, one-way ANOVA, multiple comparison using the Tukey test. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. The levels of nucleoside modifications were normalized to the total molar amount of canonical nucleosides N (N = C + U + G + A). Source data are available online for this figure.
To further elucidate the dynamics of m6A(m) upon dysregulation of FTO, we analyzed poly(A) RNA from FTO KO cells. We observed a small but significant increase in both m6A and m6Am levels in FTO KO compared to WT 293T cells when combining nine independent measurements (Fig. 3D), which is consistent with a previous report (Wei et al. 2018). This m6A and m6Am stabilization in mRNAs had no impact on the level of inosine in mRNAs (Fig. 3D). We observed a slight reduction in m7G levels in FTO KO cells (Fig. 3D) but this could be due to slightly higher tRNA or rRNA contamination in the WT poly(A) RNA samples, as indicated by m5U levels (Supplemental Fig. S3C), even though this contamination did not affect the levels of m6A (Fig. 3D). In summary, FTO primarily targets cap-linked m6Am in total RNA, but also has a relatively minor yet significant effect on m6A and m6Am levels in poly(A) RNA of 293T cells. These results suggest that m6Am modification displays a dynamic potential in steady-state levels of total RNA, whereas the effects on poly(A) RNA are rather small in this cell line.
ALKBH5 depletion has little impact on m6A levels in steady-state mRNAs
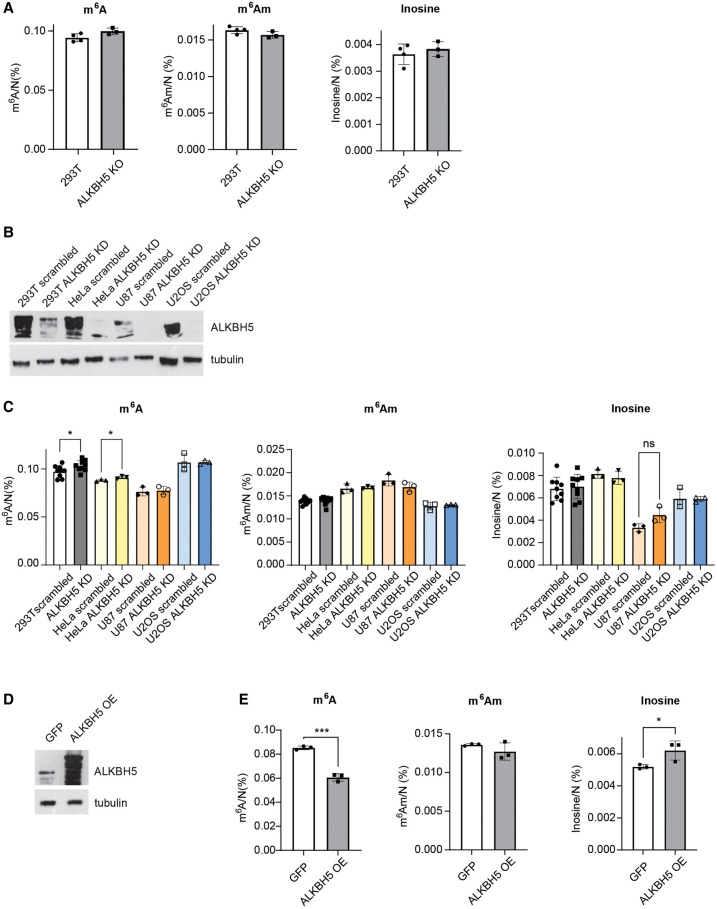
ALKBH5, a conserved m6A RNA demethylase found in most eukaryotes, is considered to be the main eraser of m6A in mRNA. To date, its activity has been linked to protein-coding transcripts (for review, see Rajecka et al. 2019). Given that FTO depletion revealed little impact on mRNA m6A stabilization, we next investigated how ALKBH5 dysregulation affects m6A and other mRNA modifications. Firstly, we compared m6A levels in poly(A) RNA in control 293T cells and ALKBH5 KO cells. Surprisingly, we observed only a very small and insignificant increase in m6A (Fig. 4A) and no changes in the other marks evaluated when comparing ALKBH5 KO cells with WT 293T (Fig. 4A; Supplemental Fig. S4A). We hypothesized that the CRISPR-generated ALKBH5 KO cells might have adapted their RNA metabolism to the lack of ALKBH5. Therefore, we performed conditional ALKBH5 depletion by siRNAs and extended the study to several other human cell lines apart from 293T cells (Fig. 4B). Similarly to FTO depletion, we detected only a very mild increase in m6A in poly(A) RNA in 293T and HeLa cells and no significant change for the other two tested cell lines (Fig. 4C). ALKBH5 downregulation did not affect levels of any of the other measured modifications (Fig. 4C; Supplemental Fig. S4C) Interestingly, the relative levels of poly(A) RNA m6A, m6Am, inosine, and m7G modifications differed between individual cell lines and these changes do not seem to be explained by differences on writer's expression between cell lines (Fig. 4C; Supplemental Fig. S4B,C). On the contrary, overexpression of ALKBH5 (Fig. 4D) led to a strong and significant decrease in m6A and small but significant increase in inosine in the poly(A) RNA fractions (Fig. 4E). The other determined RNA modifications as well as protein level of methyltransferases remained unaffected (Supplemental Fig. S4D,E).
FIGURE 4.
ALKBH5 depletion has a minor effect on m6A levels in poly(A) RNA. (A) LC-MS/MS measurements of m6A, m6Am, and inosine in poly(A) RNAs isolated from WT and ALKBH5 KO cells. (B) Immunoblot analysis of protein expression in 293T, HeLa, U87, and U2OS cells transfected with control (scrambled) or ALKBH5 targeting siRNAs (ALKBH5 KD). The levels of proteins were detected with specific antibodies listed in Materials and Methods. (C) LC-MS/MS measurements of m6A, m6Am, and inosine in poly(A) RNAs isolated from WT and ALKBH5 KD in 293T, HeLa, U87, and U2OS cells. 293T (n = 9), HeLa, U87, and U2OS (n = 3). (D) Immunoblot analysis of protein expression in 293T cells overexpressing ALKBH5 (ALKBH5 OE) or GFP control. The levels of proteins were detected with specific antibodies listed in Materials and Methods. (E) LC-MS/MS measurements of m6A, m6Am, and inosine in poly(A) RNAs isolated from control (GFP) and ALKBH5 overexpressing cells. Data are mean ± SD, one-way ANOVA, multiple comparison with the Tukey test (for ALKBH5 KD). Data are mean ± SD, unpaired two-tailed t-test (for ALKBH5 KO and ALKBH5 OE). (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. The levels of nucleoside modifications were normalized to the total molar amount of canonical nucleosides N (N = C + U + G + A). Source data are available online for this figure.
In summary, ALKBH5 depletion demonstrated minimal to no effect, exerting a nearly zero effect on m6A levels in a steady-state pool of mRNAs, with notable cell-type-dependent variations. Only strong upregulation of ALKBH5 shows a strong m6A eraser effect.
DISCUSSION
The aim of this study was to investigate the dynamics of m6A, m6Am, and inosine and to examine whether levels of m6A and inosine, the two RNA modifications that are among the most prevalent in mRNA, influence each other. Both of these modifications target the same position on the adenosine, so they are mutually exclusive. However, they occupy distinct sequence-specific positions and RNA structures, precluding direct exchange of the modifications at the same position. Consequently, any potential changes in m6A or inosine levels would be indirect. We chose LC-MS/MS to perform the analysis as we want to observe the global levels of m6A, m6Am, and inosine upon dysregulation of the enzymes involved in generating or removing these modifications. Analyzing different modifications in parallel in the same samples should mean that any artifact introduced by this method would have an effect on both modifications, limiting the potential biases of the analysis.
In our study, we initially focused on the establishment of an LC-MS/MS method that would allow us to measure cap-linked and internal modifications. Previous studies have quantified cap-linked dinucleotides upon NP1 RNA digestion (Mauer et al. 2019; Wang et al. 2019; Galloway et al. 2020) or used NP1 together with a pyrophosphatase for cleavage to single nucleotides (Boulias et al. 2019; Sendinc et al. 2019). We established a method that allowed us to distinguish between internal and cap-linked modifications by combining the NP1 and SVP digestion. The measurement of RNAs digested by NP1 alone or in combination with SVP enabled the identification of cap-linked modifications. Secondly, previous mass spectrometry studies on m6A in poly(A) RNA did not address the potential significant contamination resulting from the ineffective isolation of poly(A) RNA from total RNA (Zheng et al. 2013; Wei et al. 2018). Consequently, we focused on the measurement of tRNA- and rRNA-specific modifications, which would allow us to monitor the levels of tRNA and rRNA contamination in each sample. By documenting the purity of the poly(A) RNA samples, we can reflect m6A levels independently of tRNA or rRNA contamination.
Our results of the m6A-inosine interconnection studies suggest that the responsible proteins, METTL3 and ADAR1, might affect the levels of the cross-related marks. This is likely to occur in a cell-type-dependent manner, as it has recently been demonstrated that the ADAR1 interactome varies in different cell types (Vukić et al. 2024). The KDs of either METTL3 or ADAR1 in 293T or HEK293 cells, respectively, did not lead to changes in the other modifications. These observations are consistent with the latest reports on the cross-regulation between METTL3 and ADAR1. Terajima et al. (2021) demonstrated that the downregulation of METTL3 does not affect ADAR1 levels in the glioblastoma A172 cell line, unless the interferon response is stimulated. HEK293T cells do have a strong interferon response, so it is not certain that adding interferon would change the level of m6A. In contrast, Li et al. (2022) observed that ADAR1 activity positively regulates METTL3 expression in breast cancer cells (MCF7 and MDA-MB-231). In that case, ADAR1 modifies the miR-532-5p seed sequence in METTL3 mRNA, leading to its stabilization, enhanced translation, and consequently to higher general m6A levels in mRNAs. It is currently unclear why a similar ADAR1-mediated regulation of METTL3 was not observed in HEK293 cells. The miR532-5p is relatively highly expressed in HEK293T cells (miRmine). Thus, it may employ different mechanisms to regulate METTL3 expression in different cell types. Our findings, when considered alongside the existing published data, indicate that the consequence of METTL3 editing is cell-type dependent at the transcriptome-wide level. Furthermore, we did not observe any clear cross-regulation between m6A, m6Am, and ADAR1.
We further focused on the investigation of m6Am as a reversible mark. The double digestion of RNA with NP1 and SVP was employed to quantify m6Am levels in RNA isolated from FTO KO cells. We detected the previously reported changes in m6Am abundance in total RNA samples (Mauer et al. 2019) and confirmed previously published data indicating that m6Am in total RNA is a primary target of FTO (Mauer et al. 2017). M6Am levels in total RNA in FTO KO cells could be restored by reintroduction of WT FTO, but not by the introduction of catalytically inactive HD mutant FTO (in α-ketoglutarate coordination site H231A, D233A). The disease-associated RQ mutant FTO (in the 2-oxoglutarate coordination site R316Q; Boissel et al. 2009) showed an m6Am abundance between that observed in 293T WT and HD mutant FTO cells. This is particularly noteworthy because our previous findings indicated that RQ mutant cells exhibit different alternative splicing patterns in specific transcripts between those observed in WT and HD mutant FTO. This suggested that the splicing changes that we observed in FTO KO 293T cells (Bartosovic et al. 2017) may be regulated by m6Am levels in snRNAs. The levels of m6Am in poly(A) RNA were significantly increased upon FTO KO, in agreement with previously published data (Wei et al. 2018). Taken together, our findings indicate that m6Am exhibits reversibility and reveals dynamic potential in both total RNA and poly(A) RNA.
In regard to the measurements of m6A, our data do not support the prevailing notion that m6A is a dynamic modification. Our findings are consistent with those of the Darnell and Jaffrey groups (Meyer and Jaffrey 2017; Darnell et al. 2018), who posit that m6A is a stable modification. Firstly, m6A levels remained almost unaffected in FTO-dysregulated cells in both total RNA and poly(A) RNA, supporting the hypothesis that FTO targets m6A in mRNAs only minimally (Mauer et al. 2017) and suggesting that m6A is rather more a stable than a dynamic RNA modification. Secondly, the depletion of ALKBH5 showed only marginal effect on m6A levels in only some cell lines that we analyzed. In particular, to achieve statistical significance for a rather small overall stabilization of m6A, we had to perform a higher number of measurements than the minimum of three replicates usually required for statistical tests. Therefore, the observed results are statistically significant, although their biological relevance is rather unclear. Our observations on the ALKBH5 demethylase activity align with those reported by other groups using LC-MS/MS measurements (Zheng et al. 2013). Only high overexpression of the eraser led to a dramatic decrease of m6A in poly(A) RNA, as observed in the aforementioned study (Zheng et al. 2013). These data are consistent with the findings of other reports on cancers that have demonstrated a correlation between elevated ALKBH5 expression in various cancer types and decreased m6A levels (Zhang et al. 2016; Chao et al. 2020; Jiang et al. 2020). The elevated levels of ALKBH5 have been identified as an oncogene that can facilitate the growth of cancer cells (Jin et al. 2022; Qu et al. 2022a). However, several studies have also demonstrated its tumor suppressor function (for review, see Qu et al. 2022b), thereby revealing its dual role in carcinogenesis. Furthermore, our findings challenge the prevailing view that ALKBH5 is primarily an m6A demethylase, as its demethylase activity has been predominantly reported when it is upregulated in cancer, although several studies have also demonstrated its demethylase function also in noncancer research (Yu et al. 2020; Gao et al. 2023). ALKBH5 KO cells and animals are viable (Bai et al. 2023; Gao et al. 2023). ALKBH5 KO mice show defects in spermatogenesis and oogenesis (Tang et al. 2018; Bai et al. 2023). This is in contrast to the original proposition that functional m6A dynamicity is essential for basic cellular functions (Jia et al. 2011; Meyer and Jaffrey 2014). ALKBH5 is capable of binding the m6A through its m6A-binding pocket (Aik et al. 2014; Feng et al. 2014), thus it remains to be determined whether ALKBH5 could act as an m6A reader rather than a demethylase.
In conclusion, the data presented suggest that m6Am is a potential dynamic reversible RNA methylation mark in both pools of steady-state total RNA (presumably snRNAs) and poly(A) RNA. However, further experiments are required to provide more definitive evidence. In contrast, m6A, which was previously believed to be a dynamic mark, demonstrated close to no reversible potential in vivo on overall steady-state poly(A) RNAs. Many studies pointed to specific roles of m6A in splicing, stability, or translation of single particular mRNAs that often occur cell and/or tissue-specifically (He and He 2021; Murakami and Jaffrey 2022). Overall, our evidence does not support the hypothesis that epitranscriptomic RNA modification in general should be dynamic and resemble epigenetic modifications.
MATERIALS AND METHODS
Human cell line manipulation
Human 293 Flp-In T-REx (293T) (Invitrogen) cells were maintained as described in Covelo-Molares et al. (2021). Human HEK293 (ATCC, CRL-3216), T-REx-HeLa cells (Invitrogen), U87, and U2OS cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in the presence of 5% CO2.
Preparation of FTO and ALKBH5 KO cell lines by CRISPR–Cas9
To create cells with disrupted expression of FTO or ALKBH5, we employed the CRISPR–Cas9 system (Ran et al. 2013). We used the following sgRNAs targeting intron 2 and exon 3 of the FTO gene: FTO-intron2: CACCgACTCGTGCCCTGGAAGCCAG; FTO-exon3: CACCgTATGTCTGCAGATTTCCCCA, and the following sgRNAs targeting exon 1 of the ALKBH5 gene: ALKBH5-exon1a: CACCgACGTCCCGGGACAACTATA; ALKBH5-exon1b: CACCgTGGACTTGAGCTTCTCACGC. The oligonucleotides were individually cloned into the vectors pSpCas9(BB)-2A-GFP (PX458) (from Feng Zhang, Addgene 48138) for FTO and pSpCas9n(BB)-2A-Puro (PX462) V2.0 (from Feng Zhang, Addgene 62987) for ALKBH5, and verified by Sanger sequencing. On the day of transfection, 250 ng of each sgRNA construct was mixed with serum-free DMEM and transfected with the Lipofectamine3000 reagent (Invitrogen L3000015) to 50% confluent cells. For FTO KO cells, 24 h posttransfection cells were single-cell sorted into a 96-well plate with FACS. For ALKBH5 KO, 24 h posttransfection puromycin (Applichem A2856) was added (final concentration 4 µg/mL) and cells were grown to 60% confluency. A second round of transfection was performed as described above. Twenty-four hours posttransfection, cells were counted and seeded at the density of 0.5 cell/well in 96-well plates.
The individual cells were verified as KO by immunoblot, RT-qPCR, and Sanger sequencing of the corresponding genomic DNA locus. Expression of proteins was analyzed in selected clones by immunoblot. FTO and ALKBH5 mutations/deletions were validated by PCR on the genomic DNA with primers FTO_Fwd: 5′TCATTCATCCATGCACAAATCC3′, FTO_Rev: 5′GCAGAGCAGCATACAACGTA3′, ALKBH5_Fwd: 5′ CCGTTGTCGCCACCGTTGCATGAC3′, and ALKBH5_Rev: 5′ AGTCCTCCTGATACTTGCGCTTGG 3′.
RNA isolation
Total RNA was isolated with TriPure reagent (Sigma T3934-100) according to the manufacturer's protocol followed by a precipitation step with 1 volume of 5M ammonium acetate (Invitrogen AM9070G) and 2.5 volumes of 100% ethanol. RNA integrity was assessed by electrophoresing an aliquot of the RNA sample on a denaturing agarose gel stained with SYBR safe (Invitrogen S33102).
Poly(A) mRNA enrichment
One milligram of total RNA was used as input for the first step of poly(A) mRNA enrichment with the PolyATtract mRNA Isolation Systems kit (Promega Z5310) and purified according to the manufacturer's protocol. Eluted poly(A)+ mRNA was precipitated with 1 volume of 5M ammonium acetate (Life Technologies) and 2.5 volumes of 100% ethanol. After mRNA recovery, the second step of poly(A) mRNA enrichment was performed with poly(A) RNA Selection kit (Lexogen 039). To increase the yield of mRNA, 3× the suggested amount of oligo(dT) magnetic beads was used. The concentration of eluted mRNA was measured by Qubit RNA HS Assay kit (Invitrogen Q32852). Typically, 500 ng–2 µg of mRNA was obtained after the two rounds of poly(A) mRNA enrichment.
LC-MS/MS quantitative analysis of RNA modifications
The analysis of RNA modifications by LC-MS/MS was performed according to the protocol developed by Thuring et al. (2016). Carbon-13 (13C)-labeled ISs were prepared from bacterial and yeast cultures. E. coli strain BL21 (DE3)-RIL was cultivated in M9 minimal medium (3.37 mM Na2HPO4, 2.20 mM KH2PO4, 0.86 mM NaCl, 0.94 mM NH4Cl, 1.00 mM MgSO4, and 0.3mM CaCl2) supplemented with Trace elements solution (final concentration—134.00 µM EDTA, 31.00 µM FeCl3, 0.62 µM ZnCl2, 0.76 µM CuCl2, 0.42 µM CoCl2, 1.62 µM H3BO3, 0.08 µM MnCl2) and 20% D-GLUCOSE-13C6 (Merck 389374). Bacteria were harvested at late exponential phase (OD600 = 1.8), centrifuged at 6000g 10 min 4°C and twice washed with cold 1× PBS. RNA was isolated with TRI Reagent (Merck T9424) according to the manufacturer's protocol followed by a precipitation step with 1 volume of 5M ammonium acetate and 2.5 volumes of 100% ethanol.
S. cerevisiae strain BY4741 was grown in 13C-labeled rich growth yeast media (OD600 = 2) (SILANTES, 111201402) supplemented with 20% glucose 13C6 (SILANTES, 302204100) at 30°C for 12–14 h with shaking at 230–270 rpm. Yeast harvested at the late exponential phase (OD600 ∼3.5), centrifuged at 3500g 10 min 4°C and washed two times with ice-cold 1×PBS. The cell pellet was resuspended in 1 mL of TRI Reagent per 10 mL of SILANTES media. The supernatant was transferred to prefill screw-capped vials with ∼250 μL volume of acid-washed beads (500 µm diameter, BIOSPEC). Cells walls were disrupted with Precellys evolution homogenizer (Bertin Technologies) 4× (2.5 min homogenization at 6500 rpm and 2.5 min sample rest on ice). Debris from the transferred suspension was removed by centrifugation at 6000g 15 min 4°C and the supernatant was used for RNA isolation as previously described.
Synthesis of N6-(methyl-d3)-2′-O-methyladenosine (m6Am-d3) standard
A mixture of 2′-O-methyl-adenosine (250 mg, 0.89 mmol) and methyl iodide-d3 (221 µL, 3.5 mmol) in 2.0 mL of N,N-dimethylacetamide (DMA) was stirred at 28°C overnight. The reaction mixture was then treated with 20 mg of celite and stirred for 10 min. The suspension was filtered, and acetone (10 mL) was added to wash the solid part which was discarded. Crude product was precipitated from the liquid by the addition of Et2O (10 mL). The suspension was sonicated, centrifuged, and liquid was separated from the solid pellet. To purify the precipitate, MeOH (1.0 mL) and Et2O (40 mL) were added to the solid pellet. The resulting suspension was sonicated, centrifuged, and the liquid was separated from the solid pellet. This process was repeated twice. Finally, Et2O (10 mL) was added to the solids and the suspension was sonicated and then centrifuged. Removal of liquid, and drying of the solid product at RT (5–10 min) under vacuum (3.0 mbar) led to the production of crude N1-(methyl-d3)-2′-O-methyladenosine as a white off solid (350 mg).
N1-(methyl-d3)-2′-O-methyladenosine was dissolved in 0.25 M NaOH solution (15 mL) and heated overnight at 80°C. The reaction mixture was subsequently cooled to RT, and pH was adjusted to 7.5 by the addition of an aqueous 10% p-toluensulfonic acid solution. Water was removed under vacuum (72 mbar) at 50°C. MeOH (50 mL) was added to the resulting solids and stirred for 10 min at 50°C. MeOH was subsequently removed under vacuum (337 mbar) at 40°C. The solids were refluxed overnight in EtOAc (50 mL). The resulting suspension was cooled at RT, and EtOAc was removed under vacuum (240 mbar) at 40°C. MeOH (1.0 mL) and Et2O (40 mL) were then added to the solids. The resulting suspension was sonicated, centrifuged, and the liquid was separated from the solid pellet. The purification process was repeated an additional four times. Finally, Et2O (10 mL) was added to the solids and the suspension was sonicated and centrifuged. Removal of liquid and drying of the solid product at RT (5–10 min) under vacuum (3.0 mbar) led to the generation of N6-(methyl-d3)-2′-O-methyladenosine (m6Am-d3) as a white off solid (150 mg crude product, 0.542 mmol, 56% yield). A fraction of the crude product was purified by preparative HPLC (A- Triethylammonium acetate 0.1 M, pH 7, B- Acetonitrile). Co-distillations with water followed by several freeze-drying from water gave off-white solid product.
RNA digestion and LC-MS/MS analysis
Two micrograms of total RNA or 750 ng of poly(A) mRNA (or 13C-labeled IS) was digested with 0.1U NP1 ( Merck N8630) and 0.3U SVP (Worthington LS003928) to detect internal and cap-linked modifications in a buffer containing 0.02 mM ZnCl2, 20 mM ammonium acetate, pH 5.0 for 2 h at 37°C. The digestion mix was supplemented with adenosine deaminase inhibitor Pentostatin (0.7 µg). Digested nucleotides were dephosphorylated by adding 0.3U SAP (Life Technologies) and 0.1 volume of 10× dephosphorylation buffer (100 mM MgCl2, 100 mM ammonium acetate, pH 9.0) for 1 h at 37°C. ISs were also added to the reaction: 50 ng of bacterial IS, 150 ng of yeast IS, and 60 pg of m6Am-d3.
Digested nucleosides were fractionated with YMC-Triart C18 column (100 × 3.0 mm I.D., S – 3 µm, 12 nm, YMC) at 35°C with HPLC Agilent 1260 infinity (Agilent). The injection volume was 10 µL (250 ng RNA). The solvent system consisted of 0.1% formic acid/water (solvent A) and acetonitrile/0.1% formic acid (solvent B). A gradient elution program was applied at a flow rate of 0.35 mL/min at 35°C as follows: 0 min, 100% solvent A; 0–15 min, (92% A, 8% B); 15–20 min, (60% A, 40% B); 20–23 min, (60% A, 40% B); subsequently, a column equilibration step was applied – 23.1–34 min (100% A). The molecular concentration of canonical nucleosides (C, U, G, A) was measured with 1260 infinity DAD detector with InfinityLab Max-Light cartridge cell, 10 mm, 1.0 μL (Agilent). The signal was analyzed at 254 nm wavelength; 4 nm bandwidth; 360 nm reference wavelength; 100 nm reference bandwidth. Eluted nucleotides were ionized in Agilent Jet stream electrospray source with the following parameters: positive mode, gas temperature 350°C, gas flow 8 L/min, Nebulizer 50 ψ, Sheat gas temperature 350°C, Sheat gas flow 12 L/min, capillary voltage (3000 V). Nucleosides were quantified with the Agilent 6460 Triple Quad Mass Spectrometer.
Downregulation of gene expression by siRNAs
SiRNAs were transfected into cells with Lipofectamine RNAiMAX (ThermoFisher 13778150) according to the manufacturer's protocol. For METTL3 KD, we used two different siRNAs targeting METTL3 CDS (CUGCAAGUAUGUUCACUAUGA[dT][dT] and AGGAGCCAGCCAAGAAAUCAA[dT][dT], Sigma) at concentration 40 nM (20 nM each siRNA). Cells were collected 96 h after transfection. For ALKBH5 KD, four different siRNAs targeting ALKBH5 CDS (ACAAGUACUUCUUCGGCGA[dT][dT], GCGCCGUCAUCAACGACUA[dT][dT], CUGAGAACUACUGGCGCAA[dT][dT], and AAGUCGGGACUGCAUAAUUAA[dT][dT], Sigma) were used at concentration 20 nM (5 nM each siRNA). Cells were collected 72 h (24 h + 48 h transfection) after transfection. For negative controls of the RNAi treatment, we used scrambled siRNA pool (ON-TARGETplus Nontargeting Control Pool, Dharmacon D-001810-10-20) and scrambled siRNA pool (MISSION siRNA Universal Negative Control #1, Sigma SIC001) at the same concentrations as the shRNAs targeting specific mRNAs.
ADAR1 KD by shRNAs
Stable human embryonic kidney cell lines HEK293 expressing the SMARTvector Inducible Lentiviral shRNA against ADAR1 (Horizon Discovery) or scrambled shRNA were generated following the protocol described in Tassinari et al. (2021). One µg/mL puromycin was used for the selection 2 days post infection. Vector expression was induced with 1 µg/mL doxycycline refreshed every 48 h. GFP+ cells were sorted after 48 h of induction, and they were maintained in DMEM supplemented with 10% FBS (Gibco-Life Technologies), 100 U/mL penicillin, and 100 µg/mL streptomycin, at 37°C in 5% CO2. Cells were collected after 96 h of doxycycline induction.
Protein overexpression in human cell lines
For protein overexpression of ALKBH5 and GFP (control), we used stable inducible cell lines previously described in Covelo-Molares et al. (2021). The cells contain stable integrated fusion versions of Strep-HA-tag-ALKBH5 and STREP-HA-tag-GFP, respectively. For protein overexpression of FTO and its catalytically inactive HD mutant (in α-ketoglutarate coordination site H231A, D233A) and the disease-associated RQ mutant (patient mutation in FTO in the 2-oxoglutarate coordination site R316Q (Boissel et al. 2009) in the FTO KO cell line, we used stable inducible cell lines previously described in Bartosovic et al. (2017). Cells contain stable integrated fusion versions of 3xFLAG-FTO-WT, 3xFLAG-FTO-HD, or 3xFLAG-FTO-RQ, respectively. Protein expression was induced with 200 ng/mL doxycycline at 50% confluency and harvested after 24 h further incubation.
Protein expression analysis
For immunoblot analyses of 293T, FTO KO, ALKBH5 KO and cells overexpressing Strep-HA-tag-GFP and Strep-HA-tag-ALKBH5, area corresponding to one 6-well was collected from 15 cm plates before proceeding to RNA isolation. For immunoblot analysis of HEK293, HeLa, U87, and U2OS ALKBH5 KD cells, an area corresponding to half of 6 wells was collected from 10 cm plates before further proceeding to RNA isolation. Thirty micrograms of total cell extract was analyzed by SDS-PAGE and immunoblot with protein-specific antibodies was performed. Antibodies were used at the following dilutions: METTL3 1:1000 (Proteintech 15073-1-AP), METTL14 1:1000 (Sigma HPA038002-100UL), METTL16 1:1000 (OriGene TA504710), PCIF1 1:1000 (Cell Signaling Technology E8B1B), FTO 1:5000 (Abcam ab126605), ALKBH5 1:3000 (Sigma HPA007196), ADAR1 1:3000 (Antibodies-online ABIN2855100), ADAR2 1:500 (Santa Cruz Sc-73409), MDA5 1:800 (Cell Signaling Technology D74E4), RIG-I 1:800 (Cell Signaling Technology D14G6), TUB1 1:8000 (Sigma T6074), and GAPDH 1:40,000 (Proteintech 60004-1-Ig).
Statistical evaluation
P-values were determined using the two-tailed unpaired t-test for comparison of two groups. One-way ANOVA. The Tukey test was used to compare more than two groups. (*) P < 0.05, (**) P < 0.01, (***) P < 0.005. Error bars represent mean ± SD
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We would like to thank Karolína Vavroušková and Leona Kledrowetzová for excellent technical support. This work was supported by the Ministry of Education, Youth, and Sports of the Czech Republic grant RNA for therapy (CZ.02.01.01/00/22_008/0004575), the program INTER-COST (LTC18052), the Czech Science Foundation (19-21829S and 22-12871S) to Š.V., (19-16963S) to L.K., (21-27329X) to M.A.O.’C., the institutional support CEITEC 2020 (LQ1601). H.C.-M. was supported by Brno City Municipality Scholarship for Talented PhD. S.S. was funded by Czech Science Foundation (20-11101S). H.C. and P.E.R.-G. acknowledge funding from the Ministry of Education, Youth and Sports (Czech Republic), program ERC CZ (LL1603).
Author contributions: S.S. established the method and performed analyzed RNA samples on LC-MS/MS. V.R. produced results concerning FTO and ALKBH5 analyses, performed KD in cell lines, isolated total RNA and mRNA for analysis, performed immunoblot analyses, and participated at data interpretation and manuscript writing. H.C.-M. initiated the project, produced results concerning FTO analyses, established the method of mRNA isolation from human cell lines, designed part of the experiments, isolated total RNA and mRNA for analysis, interpreted the results, and participated in manuscript writing. K.S. established the method, produced results concerning ADAR1 KD, performed KD in cell lines, and isolated mRNA for analysis. K.B. performed analyzed RNA samples on LC-MS/MS. M.D. prepared and validated the ALKBH5 KO cell line. L.K. prepared and validated the FTO KO cell line. P.E.R-G. synthesized N6-(methyl-d3)-2′-O-methyladenosine (m6Am-d3) standard. H.C. supervised the synthesis of the m6Am-d3 standard. L.P.K. edited the manuscript. M.A.O.’C. designed experiments and edited the manuscript. Š.V. designed experiments, interpreted the data, and wrote the manuscript.Competing interest statement: This work does not involve any conflict of interest.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.080324.124.
Freely available online through the RNA Open Access option.
MEET THE FIRST AUTHORS
Stanislav Stejskal.

Veronika Rájecká.

Helena Covelo-Molares.

Meet the First Author(s) is an editorial feature within RNA, in which the first author(s) of research-based papers in each issue have the opportunity to introduce themselves and their work to readers of RNA and the RNA research community. Stanislav Stejskal, Veronika Rájecká, and Helena Covelo-Molares are co-first authors of this paper, “Global analysis by LC-MS/MS of N6-methyladenosine and inosine in mRNA reveal complex incidence.” Stanislav (S.S.) is currently working outside academia, specializing in mass spectrometry. His expertise includes biochemistry, RNA analysis, and data interpretation. Veronika (V.R.) is a PhD student at the Central European Institute of Technology (CEITEC). Her research focuses on the role of RNA modifications in the regulation of pre-mRNA processing. Helena (H.C.M.) is a postdoc at the Center for Research in Molecular Medicine and Chronic Diseases (CiMUS) at the Universidade de Santiago de Compostela (USC) in Galicia, Spain. Her research focuses on studying the role of RNA modifications in cell fate decisions and the cellular decline associated with aging.
What are the major results described in your paper and how do they impact this branch of the field?
This paper makes several key contributions that we believe will significantly benefit the epitranscriptomics community: (1) providing new insights into the ongoing debate on the dynamics of mRNA methylation by showing that m6A levels in steady-state mRNAs are largely unaffected by the depletion of known demethylases, (2) providing an accessible methodological approach for quantification of both internal and cap-linked m6Am modifications, and (3) highlighting the need to monitor for “contaminant” noncoding RNAs in mRNA samples analyzed by HPLC-MS/MS.
What led you to study RNA or this aspect of RNA science?
SS: I have always loved both chemistry and biology, and studying RNA allows me to explore this fascinating molecule from both perspectives.
VR: I moved from epigenetics to RNA biology, and it amazes me how different RNA and its “marks” are from DNA and how they play a big role in the cell processes.
HCM: I became interested in RNA biology, particularly RNA modifications, because I find it fascinating how malleable RNA metabolism can be. I am still amazed by how these “small marks” on RNA molecules can be interpreted by the cellular machinery to fine-tune the intricate complexity of RNA life.
What are some of the landmark moments that provoked your interest in science or your development as a scientist?
SS: A natural curiosity and the opportunity to contribute to the development of new discoveries.
VR: Interest in finding out how things work in nature and the opportunity to find new things every day.
If you were able to give one piece of advice to your younger self, what would that be?
SS: Focus first on the method, understand its limitations, and only then proceed with the measurements.
VR: There is no such thing as negative results; it is just your point of view. Try to make the best of it.
HCM: You can learn just as much from “negative results” as from “positive results” (or even more from the former!).
Are there specific individuals or groups who have influenced your philosophy or approach to science?
SS: Mary O'Connell and Liam Keegan. Thanks to them, I was able to rediscover my love for science and overcome the fear of discussing my opinions and results.
VR: Štěpánka Vaňáčová. To think outside the box and being critical not only of published results but also of my own.
HCM: I was lucky to learn from Štěpánka Vaňáčová, my PhD supervisor, how to approach science with critical thinking and an open mind. You plan your experiments with an expectation of what the results might look like, but often, the outcomes are unexpected!
What are your subsequent near- or long-term career plans?
SS: To gain greater knowledge in the field of mass spectrometry and to better delve into the mysteries of mRNA analysis, which is becoming increasingly popular due to mRNA vaccines.
HCM: I know I want to continue working in the RNA biology field. While I have spent my entire career so far in academia, I can also see myself transitioning to nonacademic positions, focusing on method development to improve the tools available for studying RNA modifications.
What were the strongest aspects of your collaboration as co-first authors?
SS: The greatest benefit is the opportunity to share results, discuss them, and collaboratively search for solutions. Additionally, someone can always take the reins of the project when another author needs to address other issues.
VR: The opportunity to critically discuss the results with Stanislav and Helena, and to share new ideas and solutions with people with different perspectives on the same questions.
HCM: I was very fortunate to work closely with Stanislav, the “MS machine expert,” and Veronika, my laboratory mate responsible for sample preparation. I think the three of us share a strong attention to detail in our bench work and a careful approach to data interpretation, which made this collaboration so fruitful.
REFERENCES
- Aik W, Scotti JS, Choi H, Gong L, Demetriades M, Schofield CJ, McDonough MA. 2014. Structure of human RNA N6-methyladenine demethylase ALKBH5 provides insights into its mechanisms of nucleic acid recognition and demethylation. Nucleic Acids Res 42: 4741–4754. 10.1093/nar/gku085 [DOI] [Google Scholar]
- Bai L, Xiang Y, Tang M, Liu S, Chen Q, Chen Q, Zhang M, Wan S, Sang Y, Li Q, et al. 2023. ALKBH5 controls the meiosis-coupled mRNA clearance in oocytes by removing the N6-methyladenosine methylation. Nat Commun 14: 6532. 10.1038/s41467-023-42302-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. 2017. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res 45: 11356–11370. 10.1093/nar/gkx778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel S, Reish O, Proulx K, Kawagoe-Takaki H, Sedgwick B, Yeo GS, Meyre D, Golzio C, Molinari F, Kadhom N, et al. 2009. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet 85: 106–111. 10.1016/j.ajhg.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulias K, Toczydlowska-Socha D, Hawley BR, Liberman N, Takashima K, Zaccara S, Guez T, Vasseur JJ, Debart F, Aravind L, et al. 2019. Identification of the m6Am methyltransferase PCIF1 reveals the location and functions of m6Am in the transcriptome. Mol Cell 75: 631–643 e638. 10.1016/j.molcel.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi V, Schillaci O, Santorsola M, Bonazza D, Broccia PV, Zappone A, Bottin C, Dell'Omo G, Kengne S, Cacchione S, et al. 2022. TGS1 mediates 2,2,7-trimethyl guanosine capping of the human telomerase RNA to direct telomerase dependent telomere maintenance. Nat Commun 13: 2302. 10.1038/s41467-022-29907-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Shang J, Ji W. 2020. ALKBH5-m6A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem Biophys Res Commun 521: 499–506. 10.1016/j.bbrc.2019.10.145 [DOI] [PubMed] [Google Scholar]
- Covelo-Molares H, Obrdlik A, Postulkova I, Dohnalkova M, Gregorova P, Ganji R, Potesil D, Gawriyski L, Varjosalo M, Vanacova S. 2021. The comprehensive interactomes of human adenosine RNA methyltransferases and demethylases reveal distinct functional and regulatory features. Nucleic Acids Res 49: 10895–10910. 10.1093/nar/gkab900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell RB, Ke S, Darnell JE Jr. 2018. Pre-mRNA processing includes N6 methylation of adenosine residues that are retained in mRNA exons and the fallacy of “RNA epigenetics”. RNA 24: 262–267. 10.1261/rna.065219.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485: 201–206. 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Levanon EY. 2018. A-to-I RNA editing—immune protector and transcriptome diversifier. Nat Rev Genet 19: 473–490. 10.1038/s41576-018-0006-1 [DOI] [PubMed] [Google Scholar]
- Feng C, Liu Y, Wang G, Deng Z, Zhang Q, Wu W, Tong Y, Cheng C, Chen Z. 2014. Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. J Biol Chem 289: 11571–11583. 10.1074/jbc.M113.546168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J, Gehlen J, Ehrenhofer-Murray AE. 2008. Hypermethylation of yeast telomerase RNA by the snRNA and snoRNA methyltransferase Tgs1. J Cell Sci 121: 3553–3560. 10.1242/jcs.033308 [DOI] [PubMed] [Google Scholar]
- Galloway A, Atrih A, Grzela R, Darzynkiewicz E, Ferguson MAJ, Cowling VH. 2020. CAP-MAP: cap analysis protocol with minimal analyte processing, a rapid and sensitive approach to analysing mRNA cap structures. Open Biol 10: 190306. 10.1098/rsob.190306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zimmer JT, Vasic R, Liu C, Gbyli R, Zheng SJ, Patel A, Liu W, Qi Z, Li Y, et al. 2023. ALKBH5 modulates hematopoietic stem and progenitor cell energy metabolism through m6A modification-mediated RNA stability control. Cell Rep 42: 113163. 10.1016/j.celrep.2023.113163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozhik AV, Olarerin-George AO, Sindelar M, Li X, Gross SS, Jaffrey SR. 2019. Antibody cross-reactivity accounts for widespread appearance of m1A in 5′UTRs. Nat Commun 10: 5126. 10.1038/s41467-019-13146-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- He PC, He C. 2021. m6A RNA methylation: from mechanisms to therapeutic potential. EMBO J 40: e105977. 10.15252/embj.2020105977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, et al. 2011. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7: 885–887. 10.1038/nchembio.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wan Y, Gong M, Zhou S, Qiu J, Cheng W. 2020. RNA demethylase ALKBH5 promotes ovarian carcinogenesis in a simulated tumour microenvironment through stimulating NF-κB pathway. J Cell Mol Med 24: 6137–6148. 10.1111/jcmm.15228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Li M, Chang H, Wang R, Zhang Z, Zhang J, He Y, Ma H. 2022. The m6A demethylase ALKBH5 promotes tumor progression by inhibiting RIG-I expression and interferon alpha production through the IKKε/TBK1/IRF3 pathway in head and neck squamous cell carcinoma. Mol Cancer 21: 97. 10.1186/s12943-022-01572-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. 2015. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev 29: 2037–2053. 10.1101/gad.269415 [DOI] [Google Scholar]
- Kellner S, Neumann J, Rosenkranz D, Lebedeva S, Ketting RF, Zischler H, Schneider D, Helm M. 2014. Profiling of RNA modifications by multiplexed stable isotope labelling. Chem Commun (Camb) 50: 3516–3518. 10.1039/c3cc49114e [DOI] [PubMed] [Google Scholar]
- Legrand C, Tuorto F, Hartmann M, Liebers R, Jacob D, Helm M, Lyko F. 2017. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res 27: 1589–1596. 10.1101/gr.210666.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang NX, Yin C, Jiang SS, Li JC, Yang SY. 2022. RNA editing enzyme ADAR1 regulates METTL3 in an editing dependent manner to promote breast cancer progression via METTL3/ARHGAP5/YTHDF1 axis. Int J Mol Sci 23: 9656. 10.3390/ijms23179656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht K, Hartl M, Amman F, Anrather D, Janisiw MP, Jantsch MF. 2019. Inosine induces context-dependent recoding and translational stalling. Nucleic Acids Res 47: 3–14. 10.1093/nar/gky1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. 2015. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12: 767–772. 10.1038/nmeth.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. 2014. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10: 93–95. 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KD. 2024. RNA binding by the m6A methyltransferases METTL16 and METTL3. Biology (Basel) 13: 391. 10.3390/biology13060391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, et al. 2017. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 541: 371–375. 10.1038/nature21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Sindelar M, Despic V, Guez T, Hawley BR, Vasseur JJ, Rentmeister A, Gross SS, Pellizzoni L, Debart F, et al. 2019. FTO controls reversible m6Am RNA methylation during snRNA biogenesis. Nat Chem Biol 15: 340–347. 10.1038/s41589-019-0231-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Jaffrey SR. 2014. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol 15: 313–326. 10.1038/nrm3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Jaffrey SR. 2017. Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol 33: 319–342. 10.1146/annurev-cellbio-100616-060758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149: 1635–1646. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Jaffrey SR. 2022. Hidden codes in mRNA: control of gene expression by m6A. Mol Cell 82: 2236–2251. 10.1016/j.molcel.2022.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK. 2017. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169: 824–835.e814. 10.1016/j.cell.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh HX, Mirza AH, Pickering BF, Jaffrey SR. 2022. Alternative splicing of METTL3 explains apparently METTL3-independent m6A modifications in mRNA. PLoS Biol 20: e3001683. 10.1371/journal.pbio.3001683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CA, Minczuk M. 2020. TRMT2B is responsible for both tRNA and rRNA m5U-methylation in human mitochondria. RNA Biol 17: 451–462. 10.1080/15476286.2020.1712544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Hou Y, Chen Q, Chen J, Li Y, Zhang E, Gu H, Xu R, Liu Y, Cao W, et al. 2022a. RNA demethylase ALKBH5 promotes tumorigenesis in multiple myeloma via TRAF1-mediated activation of NF-κB and MAPK signaling pathways. Oncogene 41: 400–413. 10.1038/s41388-021-02095-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Yan H, Hou Y, Cao W, Liu Y, Zhang E, He J, Cai Z. 2022b. RNA demethylase ALKBH5 in cancer: from mechanisms to therapeutic potential. J Hematol Oncol 15: 8. 10.1186/s13045-022-01224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecka V, Skalicky T, Vanacova S. 2019. The role of RNA adenosine demethylases in the control of gene expression. Biochim Biophys Acta Gene Regul Mech 1862: 343–355. 10.1016/j.bbagrm.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengaraj P, Obrdlik A, Vukic D, Varadarajan NM, Keegan LP, Vanacova S, O'Connell MA. 2021. Interplays of different types of epitranscriptomic mRNA modifications. RNA Biol 18: 19–30. 10.1080/15476286.2021.1969113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter F, Plehn JE, Bessler L, Hertler J, Jorg M, Cirzi C, Tuorto F, Friedland K, Helm M. 2021. RNA marker modifications reveal the necessity for rigorous preparation protocols to avoid artifacts in epitranscriptomic analysis. Nucleic Acids Res 50: 4201–4215. 10.1093/nar/gkab1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponara AG, Enger MD. 1969. Occurrence of N2,N2,7-trimethylguanosine in minor RNA species of a mammalian cell line. Nature 223: 1365–1366. 10.1038/2231365a0 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Pollex T, Hanna K, Lyko F. 2009. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res 37: e12. 10.1093/nar/gkn954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, et al. 2014a. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159: 148–162. 10.1016/j.cell.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. 2014b. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep 8: 284–296. 10.1016/j.celrep.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. 1998. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev 26: 217–229. 10.1016/s0165-0173(97)00062-3 [DOI] [PubMed] [Google Scholar]
- Sendinc E, Valle-Garcia D, Dhall A, Chen H, Henriques T, Navarrete-Perea J, Sheng W, Gygi SP, Adelman K, Shi Y. 2019. PCIF1 catalyzes m6Am mRNA methylation to regulate gene expression. Mol Cell 75: 620–630.e9. 10.1016/j.molcel.2019.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia K, Wiatrek D, Khan A, Michalik D, Sambrani N, Sedmik J, Vukic D, O'Connell MA, Keegan LP. 2019. ADAR RNA editing in innate immune response phasing, in circadian clocks and in sleep. Biochim Biophys Acta Gene Regul Mech 1862: 356–369. 10.1016/j.bbagrm.2018.10.011 [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al. 2023. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat Commun 14: 6523. 10.1038/s41467-023-42025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Klukovich R, Peng H, Wang Z, Yu T, Zhang Y, Zheng H, Klungland A, Yan W. 2018. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3′-UTR mRNAs in male germ cells. Proc Natl Acad Sci 115: E325–E333. 10.1073/pnas.1717794115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari V, Cesarini V, Tomaselli S, Ianniello Z, Silvestris DA, Ginistrelli LC, Martini M, De Angelis B, De Luca G, Vitiani LR, et al. 2021. ADAR1 is a new target of METTL3 and plays a pro-oncogenic role in glioblastoma by an editing-independent mechanism. Genome Biol 22: 51. 10.1186/s13059-021-02271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima H, Lu M, Zhang L, Cui Q, Shi Y, Li J, He C. 2021. N6-methyladenosine promotes induction of ADAR1-mediated A-to-I RNA editing to suppress aberrant antiviral innate immune responses. PLoS Biol 19: e3001292. 10.1371/journal.pbio.3001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terns MP, Dahlberg JE. 1994. Retention and 5′ cap trimethylation of U3 snRNA in the nucleus. Science 264: 959–961. 10.1126/science.8178154 [DOI] [PubMed] [Google Scholar]
- Thuring K, Schmid K, Keller P, Helm M. 2016. Analysis of RNA modifications by liquid chromatography-tandem mass spectrometry. Methods 107: 48–56. 10.1016/j.ymeth.2016.03.019 [DOI] [PubMed] [Google Scholar]
- Torres AG, Pineyro D, Rodriguez-Escriba M, Camacho N, Reina O, Saint-Leger A, Filonava L, Batlle E, Ribas de Pouplana L. 2015. Inosine modifications in human tRNAs are incorporated at the precursor tRNA level. Nucleic Acids Res 43: 5145–5157. 10.1093/nar/gkv277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukić D, Cherian A, Keskitalo S, Bong Yih T, Marônek M, Yadav L, Keegan LP, Varjosalo M, O'Connell MA. 2024. Distinct interactomes of ADAR1 nuclear and cytoplasmic protein isoforms and their responses to interferon induction. Nucleic Acids Res 52: 14184–14204. 10.1093/nar/gkae1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Alvin Chew BL, Lai Y, Dong H, Xu L, Balamkundu S, Cai WM, Cui L, Liu CF, Fu XY, et al. 2019. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res 47: e130. 10.1093/nar/gkz751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhang J, Lin X, Yang L, Zhou Q, Mi X, Li Q, Wang S, Li D, Liu XM, et al. 2023. METTL16 promotes translation and lung tumorigenesis by sequestering cytoplasmic eIF4E2. Cell Rep 42: 112150. 10.1016/j.celrep.2023.112150 [DOI] [PubMed] [Google Scholar]
- Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hobartner C, Sloan KE, Bohnsack MT. 2017. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep 18: 2004–2014. 10.15252/embr.201744940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, Shi H, Cui X, Su R, Klungland A, et al. 2018. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell 71: 973–985 e975. 10.1016/j.molcel.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurth L, Gribling-Burrer AS, Verheggen C, Leichter M, Takeuchi A, Baudrey S, Martin F, Krol A, Bertrand E, Allmang C. 2014. Hypermethylated-capped selenoprotein mRNAs in mammals. Nucleic Acids Res 42: 8663–8677. 10.1093/nar/gku580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang JF, Yang Q, Liu CX, Wu M, Chen LL, Yang L. 2018. N6-methyladenosines modulate A-to-I RNA editing. Mol Cell 69: 126–135.e6. 10.1016/j.molcel.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Yoshinaga M, Han K, Morgens DW, Horii T, Kobayashi R, Tsuruyama T, Hia F, Yasukura S, Kajiya A, Cai T, et al. 2022. The N6-methyladenosine methyltransferase METTL16 enables erythropoiesis through safeguarding genome integrity. Nat Commun 13: 6435. 10.1038/s41467-022-34078-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Shen L, Liu Y, Ming H, Zhu X, Chu M, Lin J. 2020. The m6A methyltransferase METTL3 cooperates with demethylase ALKBH5 to regulate osteogenic differentiation through NF-κB signaling. Mol Cell Biochem 463: 203–210. 10.1007/s11010-019-03641-5 [DOI] [PubMed] [Google Scholar]
- Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL. 2016. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci 113: E2047–E2056. 10.1073/pnas.1602883113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, et al. 2013. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49: 18–29. 10.1016/j.molcel.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Corovic M, Hoch-Kraft P, Meiser N, Mesitov M, Kortel N, Back H, Naarmann-de Vries IS, Katti K, Obrdlik A, et al. 2024. m6A sites in the coding region trigger translation-dependent mRNA decay. Mol Cell 84: 4576–4593.e12. 10.1016/j.molcel.2024.10.033 [DOI] [PubMed] [Google Scholar]