Abstract
Background
Tenofovir alafenamide (TAF)-based antiretroviral therapy (ART) regimens have been associated with adverse changes in lipid and glucose profiles compared with tenofovir disoproxil fumarate (TDF)-based ART, but data in pregnancy are limited. We evaluated metabolic markers in pregnant women with human immunodeficiency virus (HIV) after starting TAF- versus TDF-based ART.
Methods
We analyzed data within the IMPAACT 2010/VESTED trial, which demonstrated better pregnancy outcomes in pregnant women randomized to initiate TAF/Emtricitabine/Dolutegravir (TAF/FTC + DTG; n = 217) or TDF/FTC + DTG (n = 215). We measured non-fasting plasma concentrations of glucose, total-cholesterol, low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), lipoprotein (a), and triglycerides from samples collected 8 weeks after enrollment. We employed linear regression models to estimate by-arm mean differences.
Results
In total, 219 participants enrolled in the DTG arms in Zimbabwe and Uganda: 109 in the TAF/FTC + DTG and 110 in the TDF/FTC + DTG arms. At study entry, mean gestational age was 22.6 weeks, median HIV-1 RNA was 711 copies/mL, and mean age was 25.8 years. By 8 weeks, mean total cholesterol was 12 mg/dL higher in women randomized to TAF/ FTC + DTG versus TDF/FTC + DTG (95% confidence interval [CI]: 3.8, 21.1). Pregnant women in the TAF/FTC + DTG arm had higher mean LDL-C (7.1 mg/dL, 95% CI: .2, 14.0), triglycerides (12.3 mg/dL, 95% CI: 1.8, 22.7), lipoprotein (a) (7.3 mg/dL, 95% CI: 1.1, 13.6), and lower mean HDL-C (2.8 mg/dL, 95% CI: .1, 5.6) compared to the TDF/FTC + DTG arm.
Conclusions
Pregnant women randomized to start TAF/FTC + DTG had higher lipids than those randomized to TDF/FTC + DTG within 8 weeks of ART initiation. However, lipid levels were within normal reference ranges.
Keywords: metabolic complications, tenofovir, pregnancy, hyperlipidemia, HIV
In pregnant women with human immunodeficiency virus (HIV), Tenofovir alafenamide (TAF)-based antiretroviral therapy (ART) showed elevated lipid levels compared to Tenofovir disoproxil fumarate (TDF)-based ART. However, levels remained within normal ranges, mitigating clinical concerns.
Each year, approximately 1.3 million women with human immunodeficiency virus (HIV) become pregnant, and approximately 80% of these women take combination antiretroviral therapy (ART) in pregnancy, which is recommended for all people with HIV [1]. ART has substantially reduced perinatal transmission of HIV and maternal morbidity and mortality in pregnant women with HIV, but some regimens have been associated with metabolic changes in lipid and glucose profiles [2, 3]. It is essential to optimize HIV treatment regimens during pregnancy for the health of both the mother and the fetus.
Epidemiologic studies have demonstrated that metabolic dysfunction and insulin resistance are associated with adverse pregnancy outcomes [4], but there are limited data on metabolic changes associated with ART in pregnant women. Understanding metabolic changes linked to ART in pregnant women with HIV is crucial for several reasons: ART-related metabolic adverse effects can increase the risk of end-organ damage to a degree comparable to traditional risk factors such as diabetes and hypertension, and the occurrence of metabolic complications may prompt antiretroviral discontinuation or switch in people on ART. Data regarding the risk of adverse metabolic effects of specific antiretrovirals in pregnancy can permit selection of the safest regimens.
The metabolic adverse effects attributable to certain ARTs (integrase strand inhibitors, nucleoside reverse transcriptase inhibitors; protease inhibitors) encompass alterations in lipid and glucose metabolism, abnormalities in fat distribution and increased insulin resistance, thereby predisposing individuals to hyperlipidemia, impaired glucose tolerance, and diabetes mellitus [2, 5]. ART has also been implicated in inducing metabolic dysfunction [2]. Tenofovir alafenamide (TAF), a frequent component of ART regimens in the United States and Europe, attains higher intracellular levels and approximately 90% lower plasma concentrations of the active drug metabolite, tenofovir compared to tenofovir disoproxil fumarate (TDF) [6, 7]. This profile maximizes the antiviral efficacy and potency of TAF while lowering the theoretical risk of renal and bone toxicity compared with TDF [8, 9]. However, TAF-based ART has also been associated with altered glucose metabolism, central obesity, less favorable changes in lipids, and insulin resistance compared to TDF-based ART [10, 11–13]. These differences may be more due to inverse relationships between TDF and weight, lipids, and glucose rather than a positive association between these outcomes and TAF [3, 14]. The IMPAACT 2010 (VESTED) 3-arm randomized ART trial found that TAF/FTC + DTG started in pregnancy had better pregnancy and neonatal outcomes compared to TDF/FTC + DTG and TDF/FTC/efavirenz and was also associated with the greatest pregnancy weight gain; higher weight gain in pregnancy was actually associated with better pregnancy outcomes in the VESTED trial [15]. Other pregnancy studies have also demonstrated greater weight gain with TAF–DTG [16–18] or with raltegravir-based ART, and better pregnancy outcomes with integrase strand inhibitor-associated weight gain [19].
In this study, we evaluated differences between TAF-based versus TDF-based ART regimens and markers of metabolic dysfunction in a subset of pregnant women enrolled in the IMPAACT 2010/VESTED trial. We hypothesized that TAF/FTC + DTG started in pregnancy would be associated with higher levels of markers of metabolic dysfunction during pregnancy compared to TDF/FTC + DTG.
METHODS
We conducted an exploratory post hoc analysis using stored samples and existing data from women who participated in the IMPAACT 2010/VESTED trial [15], a phase III, open-labeled randomized controlled trial of pregnant women with HIV who were initiated on TAF-based versus TDF-based ART during pregnancy (NCT03048422) [15, 20]. Six hundred and forty-three ART naïve pregnant women with HIV were randomly assigned to initiate oral once-daily ART with TAF/FTC + DTG (n = 217); TDF/FTC + DTG (n = 215); or TDF/FTC/EFV (n = 211) between 14 and 28 weeks’ gestation and were followed through 50 weeks postpartum. Women enrolled at 22 sites in 9 countries (Botswana, Brazil, India, South Africa, Tanzania, Thailand, Uganda, the USA, and Zimbabwe).
For this analysis, 240 pregnant women who enrolled at sites in Zimbabwe (166 women in 3 sites) and Uganda (74 women in 2 sites) were assessed for inclusion. Women from Uganda and Zimbabwe were included because these 2 countries had the highest enrollment of participants into the IMPAACT 2010/VESTED trial, collectively recruiting 56% of all VESTED trial participants. This selection ensured a sufficient and cost-effective sample size for our study. Samples were taken from study week 8, a timepoint that would be expected to detect drug-induced changes in glucose and lipid concentrations. We excluded women with metabolic syndrome, multiple gestations, polycystic ovarian syndrome, chronic hypertension, impaired glucose tolerance, or diabetes mellitus at baseline. We excluded 3 women (due to predefined criteria) and 18 additional women whose samples were insufficient for testing, leaving 219 women (157 women from Zimbabwe and 62 women from Uganda) with bio-samples eligible for testing. The Institutional Review Board (IRB) of Johns Hopkins University and the respective IRBs of Uganda and Zimbabwe obtained the study's approval.
Covariates
We extracted maternal and neonatal data including maternal age, gestational age at ART initiation, gestational age at delivery, race/ethnicity, maternal weight at study initiation, body mass index, maternal weight gain during pregnancy, sexually transmitted infections (other than HIV), systolic and diastolic blood pressures, gestational hypertension, history of pre-eclampsia, preterm birth, preterm premature rupture of fetal membranes (PPROM), oligohydramnios, and intrauterine fetal death. Stored plasma obtained 8 weeks after enrollment were analyzed to measure low-density lipoprotein cholesterol (LDL-C), total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, lipoprotein (a), and glucose concentrations. Remnant cholesterol was calculated as (total cholesterol minus HDL-C minus LDL-C). Samples were not collected after fasting (ie, were random). The outcomes in our analysis included ART-associated total serum cholesterol concentrations, serum HDL-C, LDL-C, triglycerides, lipoprotein (a), remnant cholesterol concentrations, and maternal and fetal pregnancy outcomes.
Laboratory Assay of Bio-samples
Samples were assayed for total cholesterol, triglycerides, LDL-C, HDL-C, and lipoprotein (a) colorimetrically with ACE reagents (ACE glucose, ACE cholesterol, ACE HDL-C, ACE LDL-C, ACE triglycerides) using a standard Axcel clinical chemistry autoanalyzer (Alfa Wassermann Diagnostic Technologies, West Caldwell, New Jersey, USA). The ACE Glucose Reagent assay produces glucose-6-phosphate and adenosine diphosphate when glucose combines with adenosine triphosphate in the presence of hexokinase and magnesium. The oxidation of glucose-6-phosphate with NAD+ to produce 6-phosphogluconate and NADH is catalyzed by glucose-6-phosphate dehydrogenase at 340 nm. The amount of glucose present in the sample has a direct correlation with the overall amount of NADH generated. Bichromatically, the rise in absorbance is observed at 340 nm. Lipoprotein (a) levels were measured in mg/dL using the MEDTEST diagnostic reagent (Med Test, Canton, MI). Details of the laboratory methods are described elsewhere [21, 22].
Statistical Analysis
Continuous variables measured at baseline and week 8 were summarized using means and standard deviations. Categorical variables measured at week 8 were summarized with counts and percentages. Week 8 variables were compared between groups using the χ2 test for categorical variables (with Fisher test employed where appropriate), and the t test for continuous week 8 variables. For the metabolic parameters, linear regression models were used to estimate unadjusted and adjusted average differences by arm.
Causal mediation analysis was used to separate the estimated effect of the treatment arms on the risk of increased lipid concentrations into 2 effects, one mediated through change in maternal weight (indirect effect) and the other not mediated through the change in maternal weight (direct effect). Adjusted linear regression models estimating the ART treatment effects on maternal weight change on lipid changes were used to estimate indirect and direct treatment effects. The proportion mediated was also estimated, which indicates how much of the by-arm risk difference for having an elevated lipid can be explained by the indirect effect of changes in maternal weight.
Linear regression models were adjusted for maternal age, gestational age at ART initiation, mean arterial blood pressure at baseline, and maternal HIV viral load at study entry. Adjustment variables were selected by considering their anticipated relationship with the study outcome. Confidence intervals were computed with 95% confidence, and P values of <.05 were considered statistically significant. STATA 16 software was used for analyses (STATA, College Station, Texas, USA).
RESULTS
A total of 219 pregnant women with HIV were included: 109 women on TAF/FTC + DTG and 110 on TDF/FTC + DTG (n = 157 from Zimbabwe [77 received TAF/FTC + DTG, 80 received TDF/FTC + DTG] and n = 62 from Uganda ([32 received TAF/FTC + DTG, 30 received TDF/FTC + DTG]). Baseline characteristics were balanced between both groups (Table 1). The mean gestational age was 23.0 weeks (standard deviation [SD] 3.7), and median human immunodeficiency virus type 1 (HIV-1) RNA was 711 copies/mL (interquartile range [IQR] 142–3715) at study entry.
Table 1.
Baseline Characteristics of Pregnant Women on TAF/FTC + DTG Versus TDF/FTC + DTG
| Variable | TAF/FTC + DTG N = 109 | TDF/FTC + DTG N = 110 |
|---|---|---|
| Maternal age in years, mean (SD) | 25.6 (5.8) | 26.1 (5.8) |
| Gestational age (in weeks) at study entry (weeks), mean (SD) | 22.7 (3.9) | 22.5 (3.7) |
| Gestational age (in weeks) at delivery, mean (SD) | 40.0 (1.8) | 39.6 (2.2) |
| Participants by country, n (%) | ||
| Zimbabwe | 77 (71) | 80 (73) |
| Uganda | 32 (29) | 30 (27) |
| HIV-1 RNA at study entry, copies/mL, median (Q1, Q3) | 815.0 (177.0, 4448.0) | 568.0 (128.0, 3663.0) |
| Log10HIV-1 RNA, copies/mL, mean (SD) | 1.4 (0.5) | 1.4 (0.2) |
| Maternal weight at study entry (kg), mean (SD) | 63.1 (10.3) | 63.6 (10.4) |
| Maternal body mass index (kg/m2), mean (SD) | 24.6 (3.9) | 24.6 (3.6) |
| Systolic blood pressure (mmHg) at study entry, mean (SD) | 109.8 (9.2) | 109.8 (12.1) |
| Diastolic blood pressure (mmHg) at study entry, mean (SD) | 65.6 (7.3) | 66.8 (7.7) |
| Mean arterial pressure at study entry, mean (SD) | 80.4(7.0) | 81.2 (8.5) |
Abbreviations: DTG, Dolutegravir; FTC, Emtricitabine; HIV-1, human immunodeficiency virus type 1; SD, standard deviation; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 2 shows participant characteristics at 8 weeks after initiating ART with TAF/FTC + DTG versus TDF/FTC + DTG, respectively, including the proportions with HIV-1 RNA < 20 copies/mL (89.9% vs 93.6%; P = .32), mean maternal weight gain (3.4 kg vs 2.7 kg; P = .10), mean systolic blood pressure (109 mmHg vs 110; P = .43), and mean diastolic blood pressure (66 vs 67; P = .58). Gestational hypertension, pre-eclampsia, sexually transmitted infections (other than HIV), and premature rupture of fetal membranes were similar in both arms.
Table 2.
Characteristics at Week 8 After Commencement of TAF/FTC + DTG Versus TDF/FTC + DTG in Pregnant Women With HIV
| Variable | TAF/FTC + DTG N = 109 | TDF/FTC + DTG N = 110 | P Value |
|---|---|---|---|
| HIV-1 RNA < = 20 copies/mL, n (%) | 98 (89.9) | 103 (93.6) | .32 |
| Maternal weight (kg) at week 8 visit, mean (SD) | 66.5 (9.7) | 66.4 (10.8) | .92 |
| Maternal weight gain (kg) at week 8 visit, mean (SD) | 3.4 (2.5) | 2.7 (3.2) | .10 |
| Body mass index (kg/m2) at week 8 visit, mean (SD) | 25.9 (3.7) | 25.6 (3.8) | .57 |
| Systolic blood pressure (mmHg) at week 8, mean (SD) | 108.8 (9.9) | 110.0 (12.9) | .43 |
| Diastolic blood pressure (mmHg) at week 8, mean (SD) | 65.9 (7.7) | 66.5 (9.0) | .58 |
| Mean arterial pressure at week 8, mean (SD) | 80.2 (7.6) | 81.0 (9.6) | .48 |
| Gestational hypertension, n (%) | 2 (1.8) | 3 (2.7) | .20 |
| Pre-eclampsia, n (%) | 0 (0) | 2 (1.8) | .50 |
| Preterm premature rupture of fetal membranes, n (%) | 5 (4.6) | 1 (0.9) | .12 |
| Any STI (other than HIV), n (%) | 2 (1.8) | 0 (0) | .25 |
| Preterm birth, n (%) | 3 (2.8) | 9 (8.2) | .14 |
| Oligohydramnios, n (%) | 0 (0) | 2(1.8) | .49 |
| Intrauterine fetal death, n (%) | 3(2.8) | 2(1.8) | .68 |
Abbreviations: DTG, Dolutegravir; FTC, Emtricitabine; HIV, human immunodeficiency virus; HIV-1, human immunodeficiency virus type 1; SD, standard deviation; STI, sexually transmitted infection; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 3 shows the lipid and glucose profiles in pregnant women with HIV in the TAF/FTC + DTG versus TDF/FTC + DTG arms. Eight weeks after starting ART, mean total cholesterol was higher in pregnant women with HIV receiving TAF/FTC + DTG (159 mg/dL) versus TDF/FTC + DTG (146 mg/dL, P = < .001), as were serum triglycerides (126.4 mg/dL vs 114.4 mg/dL, P = .02), LDL-C (82.1 mg/dL vs 74.6 mg/dL, P = .03), HDL-C (51.7 mg/dL vs 48.8 mg/dL, P = .03), remnant cholesterol (25.3 mg/dL vs 22.8 mg/dL, P = .02), and lipoprotein (a) concentrations (32.3 mg/dL vs 24.7 mg/dL, P = .02). The mean blood glucose concentration 8 weeks after starting ART in pregnant women with HIV on TAF/FTC + DTG (71.4mg/dL) versus TDF/FTC + DTG (76.6 mg/dL) were not statistically significant.
Table 3.
Lipid and Glucose Profiles at Week 8 After Commencement of TAF/FTC + DTG Versus TDF/FTC + DTG in Pregnant Women With HIV
| Variable | TAF/FTC + DTG N = 109 | TDF/FTC + DTG N = 110 |
|---|---|---|
| Total cholesterol (mg/dL) at week 8, mean (SD) | 159.1 (36.1) | 146.2 (28.1) |
| LDL-C (mg/dL) at week 8, mean (SD) | 82.1 (27.9) | 74.6 (23.6) |
| HDL-C (mg/dL) at week 8, mean (SD) | 51.7 (11.6) | 48.8 (9.1) |
| Triglycerides (mg/dL) at week 8, mean (SD) | 126.4 (45.3) | 114.4 (32.2) |
| Blood glucose concentration (mg/dL) at week 8, mean (SD) | 71.4 (17.7) | 76.6 (21.6) |
| Lipoprotein (a) (mg/dL) at week 8, mean (SD) | 32.3 (27.9) | 24.7 (18.6) |
| Remnant cholesterol (mg/dL) at week 8, mean (SD) | 25.3 (9.1) | 22.8 (6.5) |
Abbreviations: DTG, Dolutegravir; FTC, Emtricitabine; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 4 shows the univariable and multivariable linear regression analyses of the estimation of differences between TAF/FTC + DTG and TDF/FTC + DTG use for lipid and glucose profiles. In the multivariable linear regression analyses, pregnant women with HIV randomized to TAF/FTC + DTG had 12 mg/dL higher mean total cholesterol compared to those randomized to TDF/FTC + DTG (β = 12.5 mg/dL, 95% CI 3.8, 21.1). Women in the TAF/FTC + DTG arm had, on average, higher mean LDL-C (β = 7.1 mg/dL, 95% CI: .2, 14.0), triglyceride (β = 12.3 mg/dL, 95% CI: 1.8, 22.7), lipoprotein (a) concentrations (β = 7.3, 95% CI: 1.1, 13.6), remnant cholesterol concentrations (β = 2.5, 95% CI: .4, 4.6), and lower mean HDL-C (β = 2.8, 95% CI: .1, 5.6) 8 weeks after starting therapy compared to women on TDF/FTC + DTG. Blood glucose concentration was, on average lower in those randomized to the TAF/FTC + DTG arm (β = −5.2, 95% CI: −10.3, .3), but the adjusted difference was not statistically significant (Table 4).
Table 4.
Univariable and Multivariable Linear Regression Models
| Univariable Linear Regression | Multivariable Linear Regression | |||||
|---|---|---|---|---|---|---|
| Average Difference Comparing TAF/FTC + DTG versus TDF/FTC + DTG | 95% Confidence Interval | P Value | Average Difference Comparing TAF/FTC + DTG versus TDF/FTC + DTG | 95% Confidence Interval | P value | |
| Total cholesterol | 13.0 | 4.4, 21.6 | <.001 | 12.5 | 3.8, 21.1 | <.001 |
| LDL-C | 7.5 | .6, 14.4 | .03 | 7.1 | .2, 14.0 | .04 |
| HDL-C | 3.0 | .2, 5.7 | .04 | 2.8 | .1, 5.6 | .04 |
| Triglycerides | 12.0 | 1.6, 22.5 | .03 | 12.3 | 1.8, 22.7 | .02 |
| Blood glucose | −5.2 | −10.4, .1 | .04 | −5.0 | −10.3, .3 | .06 |
| Lp(a) | 7.6 | 1.3, 13.9 | .02 | 7.3 | 1.1, 13.6 | .02 |
| Remnant cholesterol | 2.5 | .4, 4.6 | .02 | 2.5 | .4, 4.6 | .02 |
Models were adjusted for maternal age, gestational age at study entry, mean arterial blood pressure at study entry, and maternal HIV viral load at study entry.
Abbreviations: DTG, Dolutegravir; FTC, Emtricitabine; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; Lp, Lipoprotein; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
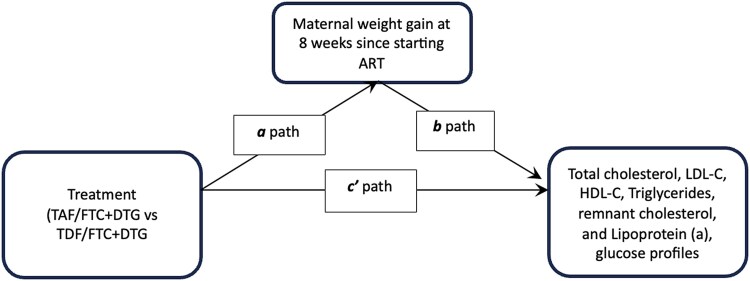
The final analysis step was determining if maternal weight change mediated the relationship between treatment with TAF/FTC + DTG or TDF/FTC + DTG and lipid changes 8 weeks after starting therapy (See Figure 1 for mediation model and labeled paths). The indirect effects are labeled as a × b, and direct effect is labeled as c′ (Figure 1). In causal mediation analysis, the percentage of the risk difference of total cholesterol, triglycerides, LDL-C, HDL-C, remnant cholesterol, and Lipoprotein (a) mediated by weight changes were 2%, 1%, 1%, 6%, 1%, and 2%, respectively. These suggest that up to 6% of observed differences in lipid changes between the randomized arms (TAF/FTC + DTG or TDF/FTC + DTG) were mediated by ART-related weight change, with other factors accounting for the remainder of the effect. The observed differences for the indirect effect were not statistically significant after accounting for maternal age, gestational age at ART initiation, mean arterial blood pressure at baseline, and baseline HIV viral load in the causal mediation regression models (see Table 5).
Figure 1.
Causal mediation model of the effect of maternal weight change on lipid and glucose profiles. Abbreviations: ART, antiretroviral therapy; DTG, Dolutegravir; FTC, Emtricitabine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Table 5.
Mediation Models of the Effect of Maternal Weight Change on Lipid Profiles
| Direct Effect (c′ path) | Indirect Effect of Maternal Weight Gain (a × b path) | |||||
|---|---|---|---|---|---|---|
| Average Difference Comparing TAF/FTC + DTG versus TDF/FTC + DTG | 95% Confidence Interval | P Value | Average Difference Comparing TAF/FTC + DTG versus TDF/FTC + DTG | 95% Confidence Interval | P Value | |
| Total cholesterol | 12.3 | 3.7, 20.8 | <.001 | 0.2 | −.8, 1.2 | .63 |
| LDL-C | 7.0 | .2, 13.9 | .04 | 0.1 | −.7, .9 | .83 |
| HDL-C | 2.6 | −.1, 5.4 | .06 | 0.2 | −.2, .5 | .35 |
| Triglycerides | 12.4 | 2.1, 22.7 | .02 | −0.1 | −1.3, 1.0 | .86 |
| Blood glucose | −5.1 | −10.6, −.1 | .04 | 0.1 | −.5, .7 | .69 |
| Lp(a) | 7.1 | 1.0, 13.3 | .02 | 0.2 | −.6, .9 | .65 |
| Remnant cholesterol | 2.5 | .4, 4.6 | .02 | 0.02 | −.3, .2 | .87 |
Models were adjusted for maternal age, gestational age at study entry, mean arterial blood pressure at study entry, and maternal HIV viral load at study entry.
Abbreviations: DTG, Dolutegravir; FTC, Emtricitabine; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; Lp, Lipoprotein; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
DISCUSSION
This study presents the first report to our knowledge of lipid and glucose metabolic profiles in pregnant women with HIV who started either TAF/FTC + DTG or TDF/FTC + DTG in pregnancy. Our study showed two noteworthy findings: first, pregnant women with HIV starting TAF/FTC + DTG had greater mean increases in total cholesterol, LDL-C, triglycerides, remnant cholesterol, and lipoprotein (a) concentrations, and lower mean decreases in HDL-C compared to TDF/FTC + DTG by 8 weeks after ART initiation. Second, the mean random blood glucose concentrations 8 weeks after starting ART in pregnant women with HIV on TAF/FTC + DTG (71.4 mg/dL) versus TDF/FTC + DTG (76.6 mg/dL) were not statistically significant. These results align with existing knowledge in non-pregnant individuals [8, 14, 23].
Our study revealed that TAF/FTC + DTG was associated with non-clinically significant increases in total cholesterol, LDL-C, HDL-C, triglycerides and lipoprotein (a) when compared to TDF/FTC + DTG. Although metabolic dysfunction such as hyperglycemia during pregnancy can lead to adverse maternal, fetal and neonatal consequences, the effects of isolated dyslipidemia in pregnant women are less clear-cut. For instance, hypertriglyceridemia has been associated with an increased risk of maternal inflammation, endothelial dysfunction and pre-eclampsia [24], but from the fetal perspective, hypertriglyceridemia can enhance non-esterified fatty acid availability for placental transfer, an essential condition for fetal growth [25]. In addition, numerous studies have demonstrated that hypertriglyceridemia and/or hypoalphalipoproteinemia were associated with increased risk for gestational diabetes [26], hypertensive disorders of pregnancy [26], preterm birth [27], fetal macrosomia, and pre-eclampsia, while concurrently decreasing the risk for small-for-gestational age fetuses [28]. Although the magnitude of hyperglyceridemia observed in these studies were similar to findings in our study, it is noteworthy that the participants in these studies were using older ART regimens. The nuanced impact of increased lipids during pregnancy and how TAF or TDF-based ART may alter these effects, remains a topic for future research.
The mechanisms underlying dyslipidemia in pregnant women with HIV on ART are intricate, involving a complex interplay of hormonal, genetic, and immunological factors [2, 29]. Studies in non-pregnant adults have demonstrated that therapy with TAF-based regimens is associated with a higher risk of dyslipidemia when compared to TDF-based regimens, prompting questions about whether dyslipidemia is an event specific to TAF or rather due to a lipid-lowering effect of TDF [30]. As a physiologically adaptive mechanism to support fetal growth in-utero, hyperlipidemia is common, particularly during the second and third trimesters of pregnancy due to increase in insulin production, heightened insulin sensitivity, increased hepatic lipase activity, reduced activity of lipoprotein lipase, and increased production of free fatty acids [31]. Previous studies have established that in a normal pregnancy, total cholesterol levels increase by approximately 50%, LDL-C by 30%–40%, HDL-C by 25%, and triglycerides by 2- to 3-fold [24]. Although there are no uniformly accepted standard reference ranges for lipids during pregnancy, the most commonly cited paper, a systematic review of approximately 70 studies, recommended the reference ranges for lipids in the second trimester as: total cholesterol (176–299 mg/dL), triglycerides (75–382 mg/dL), HDL-C (52–87 mg/mL), and LDL-C (77–184 mg/mL); whereas in the third trimester, total cholesterol ranges from 219–349 mg/dl, triglycerides from 131–453 mg/dl, HDL-C from 48–87 mg/ml, and LDL-C from 101–224 mg/mL [32]. Although we found that TAF/FTC + DTG was associated with higher increases in lipid concentrations than TDF/FTC + DTG, the increased lipid concentrations were still within the normal reference ranges for pregnancy. Other studies have demonstrated significantly higher levels of triglycerides, total cholesterol, LDL-C, and lower levels of HDL-C in pregnant women with HIV on ART compared to non-pregnant women with HIV [33]. Given that better pregnancy, neonatal and infant outcomes were observed with maternal TAF/FTC + DTG than with other regimens in the IMPAACT 2010/VESTED trial, we are not sure what mechanisms would explain the higher mean lipid concentrations with TAF/FTC + DTG. Nevertheless, the observed lipid concentrations are within the normal reference ranges for pregnancy, but more studies are needed to understand the implications of these findings for pregnancy outcomes in women with HIV who receive TAF/FTC + DTG or TDF/FTC + DTG for the entirety of their pregnancies.
This analysis has several strengths. It was embedded in the VESTED trial, the largest randomized trial of pregnant women with HIV receiving TAF- versus TDF-based ART regimens to be completed; the sample size was sufficient to provide precise estimates of differences between regimens; and prospective follow-up with high retention made the assessment of a causal relationship between regimen and biomarkers of metabolic disease feasible. Our findings are novel—a prior systematic review that included 17 studies of pregnant individuals with HIV on ART found no articles reporting maternal serum lipid concentrations and pregnancy outcomes in pregnant women with HIV on TDF or TAF-based regimens [34]. Our study also had limitations. First, generalizability may be affected because all participants in this analysis were women of African descent from 2 countries. Second, we measured outcomes after 8 weeks of antepartum follow-up and could not describe the trajectory of these metabolic outcomes postpartum nor their longer-term clinical implications. In addition, women were tested at different gestational ages (as they enrolled from 14 to 28 weeks’ gestation). The blood samples were non-fasting (due to practical considerations involving obtaining fasting glucose samples in pregnant women). Incorporation of fasting samples in future studies could further enhance the precision of metabolic measurements. Finally, we could not diagnose metabolic syndrome, as waist/hip circumference was not collected as part of the VESTED trial and is a component of most diagnostic criteria for metabolic syndrome (eg, NCEP-ATP III, EGIR, AACE, IDF, AHA/NHLBI, WHO) [35].
In conclusion, TAF/FTC + DTG started in pregnancy was associated with more significant increases in lipid concentrations by 8 weeks after starting ART, compared to TDF/FTC + DTG, but the magnitude of the differences does not appear clinically meaningful. However, our understanding of how (and to what extent) dyslipidemia and insulin resistance develop in the setting of HIV infection during pregnancy in women taking TAF-based therapies is incomplete. Further research is needed to fully elucidate the pathophysiology of metabolic disease in pregnant women with HIV, with particular emphasis on defining the roles of tenofovir-based ART.
Contributor Information
Ahizechukwu C Eke, Division of Maternal Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Sean S Brummel, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Muktar H Aliyu, Department of Health Policy and Vanderbilt Institute for Global Health, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Lynda Stranix-Chibanda, Child and Adolescent Health Unit, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe; Faculty of Medicine and Health Sciences, University of Zimbabwe Clinical Trials Research Centre, Harare, Zimbabwe.
George U Eleje, Department of Obstetrics and Gynecology, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Anambra State, Nigeria.
Ifeanyichukwu U Ezebialu, Department of Obstetrics and Gynecology, Chukwuemeka Odumegwu Ojukwu University Teaching Hospital, Amaku, Anambra State, Nigeria.
Violet Korutaro, Baylor College of Medicine Children's Foundation Uganda, Kampala, Uganda.
Deo Wabwire, Makerere University—Johns Hopkins University Research Collaboration, Kampala, Uganda.
Allen Matubu, Faculty of Medicine and Health Sciences, University of Zimbabwe Clinical Trials Research Centre, Harare, Zimbabwe.
Tapiwa Mbengeranwa, Faculty of Medicine and Health Sciences, University of Zimbabwe Clinical Trials Research Centre, Harare, Zimbabwe.
Nahida Chakhtoura, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA.
Lameck Chinula, Division of Global Women's Health, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; Department of Obstetrics and Gynecology, UNC Project Malawi, Lilongwe, Malawi.
Katie McCarthy, FHI 360, Durham, North Carolina, USA.
Kevin Knowles, Frontier Science Foundation, Amherst, New York, USA.
Chelsea Krotje, Frontier Science Foundation, Amherst, New York, USA.
Macrae F Linton, Division of Cardiovascular Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kelly E Dooley, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Paul E Sax, Division of Infectious Diseases, Brigham and Women's Hospital, Boston, Massachusetts, USA.
Todd Brown, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Shahin Lockman, Division of Infectious Diseases, Brigham and Women's Hospital, Boston, Massachusetts, USA; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Botswana Harvard Health Partnership, Gaborone, Botswana.
IMPAACT 2010/VESTED Study Team:
Sharon Nachman, James McIntyre, David P Harrington, Catherine Hill, Steven Joffe, Alwyn Mwinga, Andrew J Nunn, Haroon Saloojee, Merlin L Robb, Jonathan Kimmelman, Graeme A Meintjes, Barbara E Murray, Stuart Campbell Ray, Haroon Saloojee, Anastasios A Tsiatis, Paul A Volberding, David Glidden, Valeria Cavalcanti Rolla, Nahida Chakhtoura, Renee Browning, Jeanna Piper, Karin Klingman, Debika Bhattacharya, Patrick Jean-Philippe, Lynne Mofenson, Sean Brummel, Lauren Ziemba, Mauricio Pinilla, Chelsea Morroni, Benjamin Johnston, Chelsea Krotje, Scott McCallister, Jean van Wyk, Mark Mirochnick, Brookie Best, Kevin Robertson, Cheryl Blanchette, Nagawa Jaliaah, Andee Fox, Frances Whalen, Kevin Knowles, William Murtaugh, Mauricio Pinilla, Yao Cheng, and Emmanuel Patras
Notes
Author Contributions. A. C. E., S. L., S. S. B., K. E. D., M. H. A., G. U. E., and I. U. E. designed this exploratory study. A. C. E. assessed and analyzed the data. A. C. E. wrote the first version of the manuscript. All co-authors reviewed, edited, commented, and helped revise the manuscript. All authors approved the final version of the manuscript.
Acknowledgments. IMPAACT 2010/VESTED Study Team: Sharon Nachman, James McIntyre, David P. Harrington, Catherine Hill, Steven Joffe, Alwyn Mwinga, Andrew J. Nunn, Haroon Saloojee, Merlin L. Robb, Jonathan Kimmelman, Graeme A. Meintjes, Barbara E. Murray, Stuart Campbell Ray, Haroon Saloojee, Anastasios A. Tsiatis, Paul A. Volberding, David Glidden, Valeria Cavalcanti Rolla, Nahida Chakhtoura, Renee Browning, Jeanna Piper, Karin Klingman, Debika Bhattacharya, Patrick Jean-Philippe, Lynne Mofenson, Sean Brummel, Lauren Ziemba, Mauricio Pinilla, Chelsea Morroni, Benjamin Johnston, Chelsea Krotje, Scott McCallister, Jean van Wyk, Mark Mirochnick, Brookie Best, Kevin Robertson, Cheryl Blanchette, Nagawa Jaliaah, Andee Fox, Frances Whalen, Kevin Knowles, William Murtaugh, Mauricio Pinilla, Yao Cheng, Emmanuel Patras.
Special thanks to Dr MacRae Linton, Ms Kimberly West, and other members of the University of Vanderbilt Medical Center Lipid Laboratory, Nashville, Tennessee, for analysis of the lipid and glucose samples.
Data sharing. Data sharing for this study is restricted due to ethical guidelines outlined in the study's informed consent documents and the IMPAACT Network's approved human subjects’ protection plan. Public access to the data could jeopardize participant confidentiality. Nonetheless, researchers interested in accessing the data, including participant information with partially identifying details, may do so upon request, subject to approval by the IMPAACT Network.
Financial support. This work was supported by the National Institutes of Health (grant numbers K23HD104517 and DP1HD115433 to A. C. E.; grant numbers K24AI150349 and AI110527 to K. E. D.) and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) Network. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1. UNAIDS . UNAIDS Info Datasheets. Available at: http://aidsinfo.unaids.org/. Accessed 1 March 2024.
- 2. da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol 2015; 4:56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA 2023; 329:63–84. [DOI] [PubMed] [Google Scholar]
- 4. Grieger JA, Bianco-Miotto T, Grzeskowiak LE, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: a prospective cohort of nulliparous women. PLoS Med 2018; 15:e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thet D, Siritientong T. Antiretroviral therapy-associated metabolic complications: review of the recent studies. HIV AIDS (Auckl) 2020; 12:507–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baxi SM, Scherzer R, Greenblatt RM, et al. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. AIDS 2016; 30:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Podany AT, Bares SH, Havens J, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32:761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eke AC, Mirochnick M, Lockman S. Antiretroviral therapy and adverse pregnancy outcomes in people living with HIV. N Engl J Med 2023; 388:344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thimm MA, Eke AC. Tenofovir, pregnancy and renal function changes in pregnant women living with HIV. AIDS 2021; 35:1319–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 11. Plum PE, Maes N, Sauvage AS, et al. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect Dis 2021; 21:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surial B, Mugglin C, Calmy A, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a Cohort Study. Ann Intern Med 2021; 174:758–67. [DOI] [PubMed] [Google Scholar]
- 13. Kauppinen KJ, Kivelä P, Sutinen J. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide significantly worsens the lipid profile in a real-world setting. AIDS Patient Care STDs 2019; 33:500–6. [DOI] [PubMed] [Google Scholar]
- 14. Eke AC, Lockman S, Mofenson LM. Antiretroviral treatment of HIV/AIDS during pregnancy. JAMA 2023; 329:1308–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lockman S, Brummel SS, Ziemba L, et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2021; 397:1276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caniglia EC, Shapiro R, Diseko M, et al. Weight gain during pregnancy among women initiating dolutegravir in Botswana. EClinicalMedicine 2020; 29–30:100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zash R, Caniglia EC, Diseko M, et al. Maternal weight and birth outcomes among women on antiretroviral treatment from conception in a birth surveillance study in Botswana. J Int AIDS Soc 2021; 24:e25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eke AC, Ramaiyer M, Eleje GU, Ezebialu IU, Aliyu MH. A systematic review and meta-analysis of maternal weight changes and pregnancy outcomes associated with integrase inhibitors and tenofovir alafenamide in pregnant women with HIV. Am J Obstet Gynecol MFM 2024; 6:101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coutinho CM, Warshaw MG, Duarte G, et al. Effects of initiating raltegravir-based versus efavirenz-based antiretroviral regimens during pregnancy on weight changes and perinatal outcomes: NICHD P1081. J Acquir Immune Defic Syndr 2022; 91:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chinula L, Ziemba L, Brummel S, et al. Efficacy and safety of three antiretroviral therapy regimens started in pregnancy up to 50 weeks post partum: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet HIV 2023; 10:e363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974; 20:470–5. [PubMed] [Google Scholar]
- 22. Henry JB. Clinical diagnosis and management by laboratory methods. Philadelphia: W.B. Saunders and Company, 1979. [Google Scholar]
- 23. Lake JE, Trevillyan J. Impact of integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr Opin HIV AIDS 2021; 16:148–51. [DOI] [PubMed] [Google Scholar]
- 24. Wiznitzer A, Mayer A, Novack V, et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol 2009; 201:482.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gutaj P, Wender-Ożegowska E, Brązert J. Maternal lipids associated with large-for-gestational-age birth weight in women with type 1 diabetes: results from a prospective single-center study. Arch Med Sci 2017; 13:753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen H, Liu X, Chen Y, He B, Cheng W. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: a prospective longitudinal cohort study. BMJ Open 2016; 6:e013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith CJ, Baer RJ, Oltman SP, et al. Maternal dyslipidemia and risk for preterm birth. PLoS One 2018; 13:e0209579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin WY, Lin SL, Hou RL, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth 2016; 16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis 2006; 185:1–11. [DOI] [PubMed] [Google Scholar]
- 30. Martini S, Maggi P, Gervasoni C, et al. Dynamics of lipid profile in antiretroviral-naïve HIV-infected patients, treated with TAF-based regimens: a multicenter observational study. Biomedicines 2022; 10:3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol 2014; 15:24–31. [DOI] [PubMed] [Google Scholar]
- 32. Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009; 114:1326–31. [DOI] [PubMed] [Google Scholar]
- 33. Guaraldi G, Stentarelli C, Da Silva AD, et al. Metabolic alterations in HIV-infected pregnant women: moving to metabolic tailoring of antiretroviral drugs. AIDS Rev 2014; 16:14–22. [PubMed] [Google Scholar]
- 34. Harmsen MJ, Browne JL, Venter F, Klipstein-Grobusch K, Rijken MJ. The association between HIV (treatment), pregnancy serum lipid concentrations and pregnancy outcomes: a systematic review. BMC Infect Dis 2017; 17:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vinluan CM, Zreikat HH, Levy JR, Cheang KI. Comparison of different metabolic syndrome definitions and risks of incident cardiovascular events in the elderly. Metabolism 2012; 61:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]