Abstract
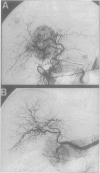
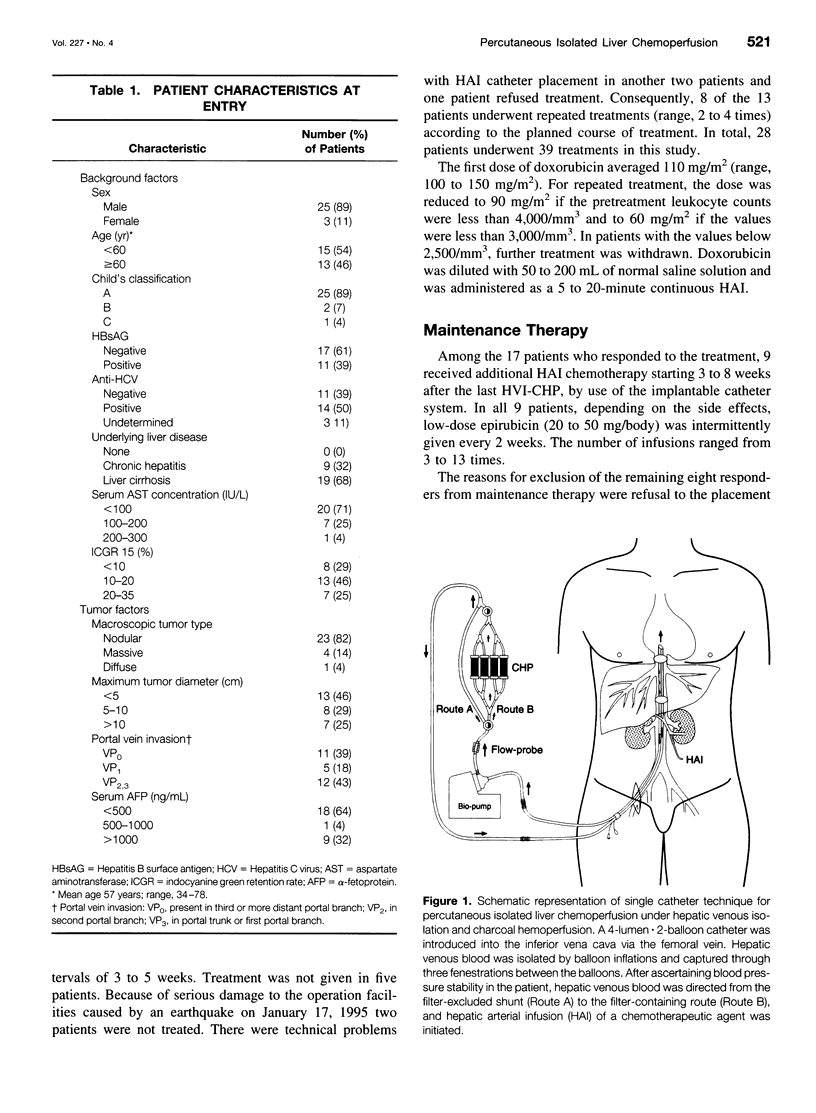
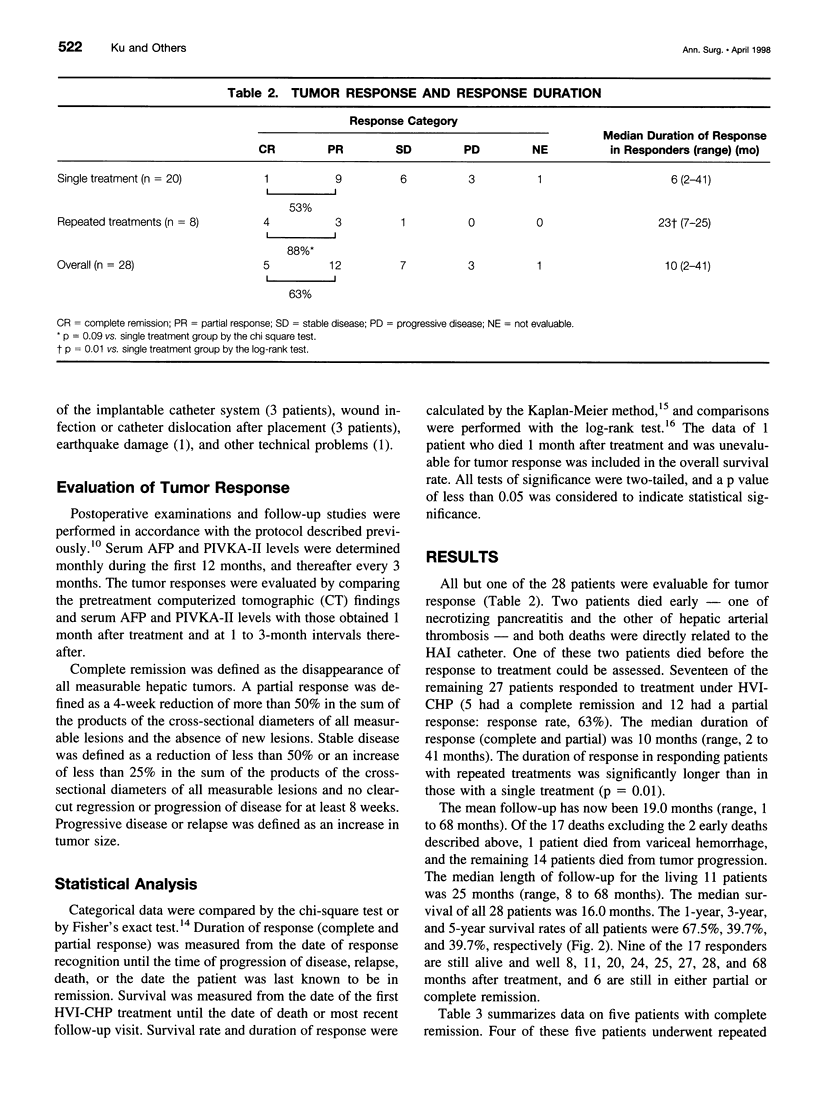
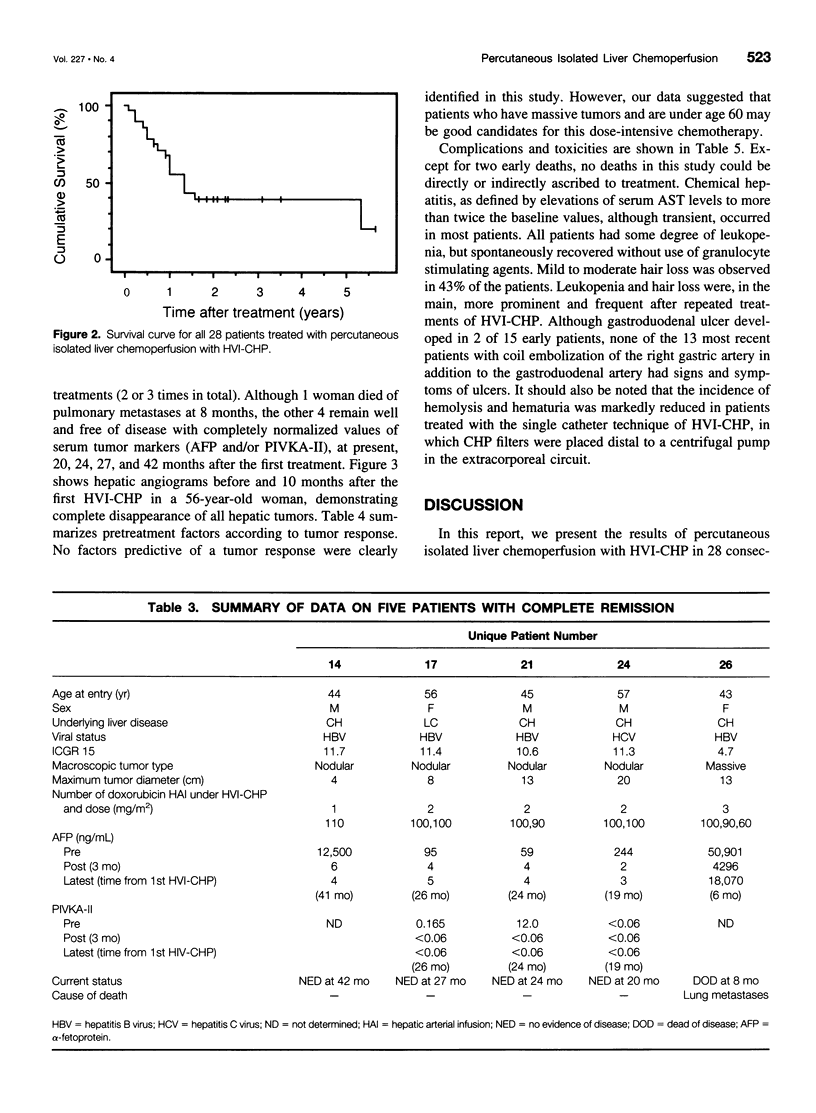
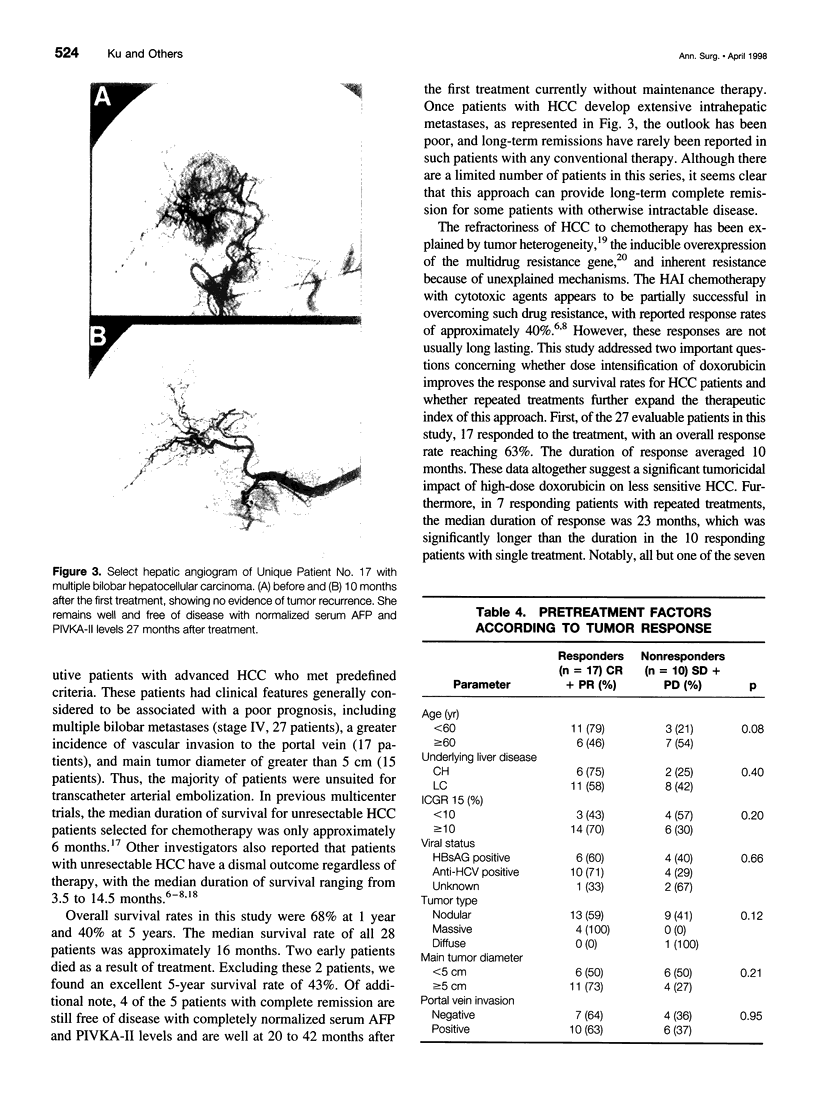
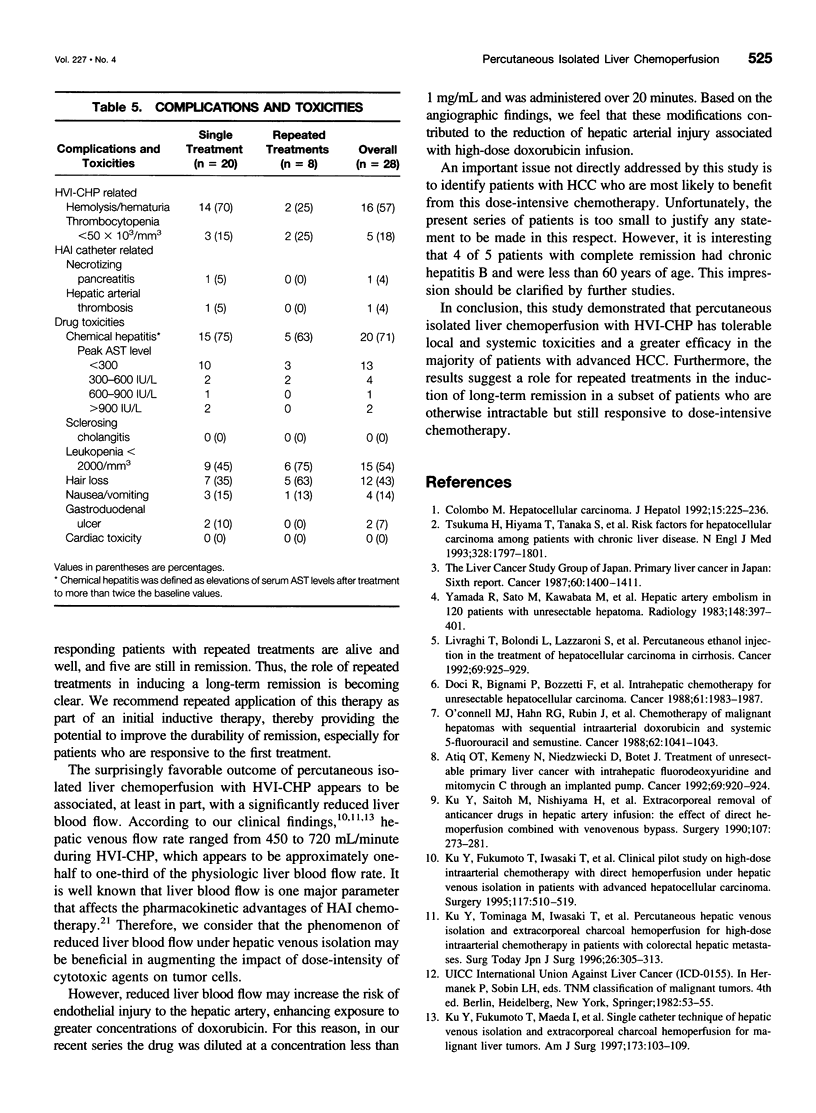
OBJECTIVE: The aim of this study was to report the long-term results of percutaneous isolated liver chemoperfusion with hepatic venous isolation and charcoal hemoperfusion (HVI-CHP) in patients with multiple advanced hepatocellular carcinoma (HCC). SUMMARY BACKGROUND DATA: The results of conventional chemotherapy including regional and systemic chemotherapy in patients with HCC remain dismal, and long-term survivors after treatment are rare among patients with multiple advanced HCC. In an effort to improve this situation, we previously developed a novel system of percutaneous isolated liver chemoperfusion with HVI-CHP. METHODS: Doxorubicin (60 to 150 mg/m2) was administered via the hepatic artery, under conditions of extracorporeal drug elimination by HVI-CHP in 28 consecutive patients with advanced HCC (39 total treatments). Hepatic venous isolation and charcoal hemoperfusion was accomplished mainly by the single catheter technique using a newly developed 4-lumen-balloon catheter, which was used to isolate and capture total hepatic venous outflow and, at the same time, to direct the filtered blood to the right atrium. RESULTS: Complete remission was achieved in five patients, of which four received repeated treatments (two or three times). Although 1 of 5 patients with complete remission died of pulmonary metastases at 8 months, the other 4 remain healthy and free of disease at 20, 24, 27, and 42 months after the first treatment. Partial responses were observed in 12 patients. Duration of response in responders (complete and partial) with repeated treatments was significantly longer than that with a single treatment (p = 0.01). The overall survival rate by the Kaplan-Meier method was 39.7% at 5 years. The treatments were well-tolerated, and the primary side effects were mild to moderate chemical hepatitis and reversible myelosuppression. CONCLUSIONS: The results suggest that percutaneous isolated liver chemoperfusion with HVI-CHP is an effective palliative treatment in the majority of patients and yields long-term complete remission in some patients with multiple advanced HCC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atiq O. T., Kemeny N., Niedzwiecki D., Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992 Feb 15;69(4):920–924. doi: 10.1002/1097-0142(19920215)69:4<920::aid-cncr2820690414>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Chen H. S., Gross J. F. Intra-arterial infusion of anticancer drugs: theoretic aspects of drug delivery and review of responses. Cancer Treat Rep. 1980 Jan;64(1):31–40. [PubMed] [Google Scholar]
- Colombo M. Hepatocellular carcinoma. J Hepatol. 1992 May;15(1-2):225–236. doi: 10.1016/0168-8278(92)90041-m. [DOI] [PubMed] [Google Scholar]
- Dexter D. L., Leith J. T. Tumor heterogeneity and drug resistance. J Clin Oncol. 1986 Feb;4(2):244–257. doi: 10.1200/JCO.1986.4.2.244. [DOI] [PubMed] [Google Scholar]
- Doci R., Bignami P., Bozzetti F., Bonfanti G., Audisio R., Colombo M., Gennari L. Intrahepatic chemotherapy for unresectable hepatocellular carcinoma. Cancer. 1988 May 15;61(10):1983–1987. doi: 10.1002/1097-0142(19880515)61:10<1983::aid-cncr2820611009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Falkson G., MacIntyre J. M., Moertel C. G., Johnson L. A., Scherman R. C. Primary liver cancer. An Eastern Cooperative Oncology Group Trial. Cancer. 1984 Sep 15;54(6):970–977. doi: 10.1002/1097-0142(19840915)54:6<970::aid-cncr2820540604>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Wu M. C., Xu G. W., Li D. Z., Cheng H., Tu Z. X., Jiang H. Q., Gu J. R. Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst. 1992 Feb 19;84(4):262–264. doi: 10.1093/jnci/84.4.262. [DOI] [PubMed] [Google Scholar]
- Ku Y., Fukumoto T., Iwasaki T., Tominaga M., Samizo M., Nishida T., Kuroda Y., Hirota S., Sako M., Obara H. Clinical pilot study on high-dose intraarterial chemotherapy with direct hemoperfusion under hepatic venous isolation in patients with advanced hepatocellular carcinoma. Surgery. 1995 May;117(5):510–519. doi: 10.1016/s0039-6060(05)80250-8. [DOI] [PubMed] [Google Scholar]
- Ku Y., Fukumoto T., Tominaga M., Iwasaki T., Maeda I., Kusunoki N., Obara H., Sako M., Suzuki Y., Kuroda Y. Single catheter technique of hepatic venous isolation and extracorporeal charcoal hemoperfusion for malignant liver tumors. Am J Surg. 1997 Feb;173(2):103–109. doi: 10.1016/S0002-9610(96)00422-9. [DOI] [PubMed] [Google Scholar]
- Ku Y., Saitoh M., Nishiyama H., Fujiwara S., Iwasaki T., Tominaga M., Maekawa Y., Ohyanagi H., Saitoh Y. Extracorporeal removal of anticancer drugs in hepatic artery infusion: the effect of direct hemoperfusion combined with venovenous bypass. Surgery. 1990 Mar;107(3):273–281. [PubMed] [Google Scholar]
- Ku Y., Tominaga M., Iwasaki T., Kitagawa T., Maeda I., Shiotani M., Kusunoki S., Maekawa Y., Samizo M., Fukumoto T. Percutaneous hepatic venous isolation and extracorporeal charcoal hemoperfusion for high-dose intraarterial chemotherapy in patients with colorectal hepatic metastases. Surg Today. 1996;26(5):305–313. doi: 10.1007/BF00311598. [DOI] [PubMed] [Google Scholar]
- Livraghi T., Bolondi L., Lazzaroni S., Marin G., Morabito A., Rapaccini G. L., Salmi A., Torzilli G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992 Feb 15;69(4):925–929. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Marcos-Alvarez A., Jenkins R. L., Washburn W. K., Lewis W. D., Stuart K. E., Gordon F. D., Kane R. A., Clouse M. E. Multimodality treatment of hepatocellular carcinoma in a hepatobiliary specialty center. Arch Surg. 1996 Mar;131(3):292–298. doi: 10.1001/archsurg.1996.01430150070014. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Hahn R. G., Rubin J., Moertel C. G. Chemotherapy of malignant hepatomas with sequential intraarterial doxorubicin and systemic 5-fluorouracil and semustine. Cancer. 1988 Sep 15;62(6):1041–1043. doi: 10.1002/1097-0142(19880915)62:6<1041::aid-cncr2820620602>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973 Sep;29(3):579–584. [PubMed] [Google Scholar]
- Tsukuma H., Hiyama T., Tanaka S., Nakao M., Yabuuchi T., Kitamura T., Nakanishi K., Fujimoto I., Inoue A., Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993 Jun 24;328(25):1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- Yamada R., Sato M., Kawabata M., Nakatsuka H., Nakamura K., Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983 Aug;148(2):397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]