Abstract
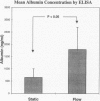
OBJECTIVE: To evaluate the survival and function of hepatocytes (HCs) on a novel three-dimensional (3D) synthetic biodegradable polymer scaffold with an intrinsic network of interconnected channels under continuous flow conditions. SUMMARY BACKGROUND DATA: The authors' laboratory has investigated HC transplantation using 3D biodegradable polymers as scaffolding as an alternative approach to treatment of end-stage liver disease. Previous studies have demonstrated survival of HCs transplanted on polymer discs in peripheral tissue sites and partial correction of single enzyme liver defects. One of the major limitations has been the insufficient survival of an adequate mass of transplanted cells; this is thought to be caused by inadequate oxygen diffusion. METHODS: HCs and nonparenchymal liver cells from Lewis rats were seeded onto 3D biodegradable polymer scaffolds. Microporous 3D polymers were created using 3D printing on copolymers of polylactide-coglycolide. The cell/polymer constructs were placed in static culture or continuous flow conditions. The devices were retrieved after 2 days and examined by scanning electron microscopy and histology. Culture medium was analyzed for albumin by enzyme-linked immunosorbent assay (ELISA). Differences in culture parameters including pH, PCO2, PO2, glucose, lactate, and HCO3 were examined. RESULTS: Scanning electron microscopy revealed successful attachment of HCs on the 3D polymer in both static and flow conditions. Histology demonstrated viable HCs in both conditions. ELISA demonstrated a significantly higher mean concentration of albumin in flow conditions than in static conditions. Culture parameter analysis revealed a significantly higher PO2 and glucose level, and a more physiologic pH in flow conditions than in static conditions. CONCLUSIONS: HCs cocultured with nonparenchymal cells can attach to and survive on the 3D polymer scaffolds in both static and flow conditions in the size and configuration used in this study. Flow conditions may provide a more conducive environment for HC metabolism and albumin synthesis than static conditions. The authors hypothesize that flow through directed channels will be necessary for the transfer of large masses of cells when implantation studies are initiated.
Full text
PDF
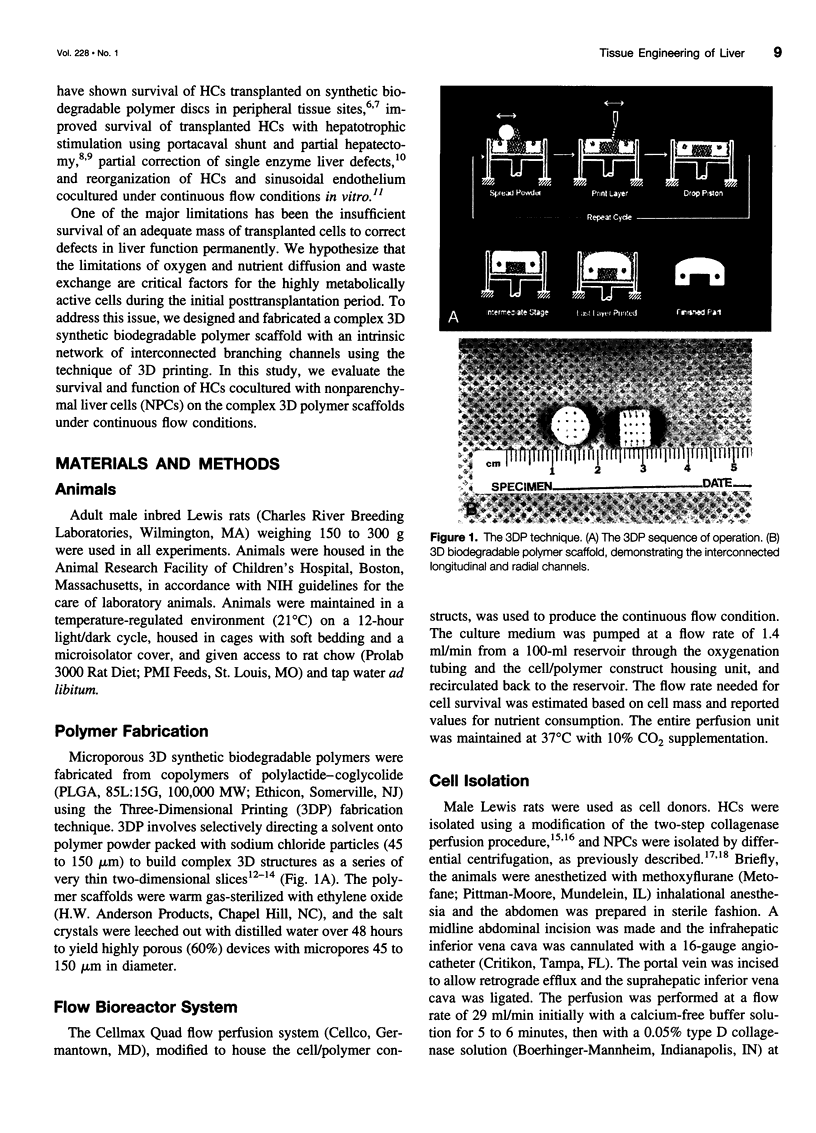

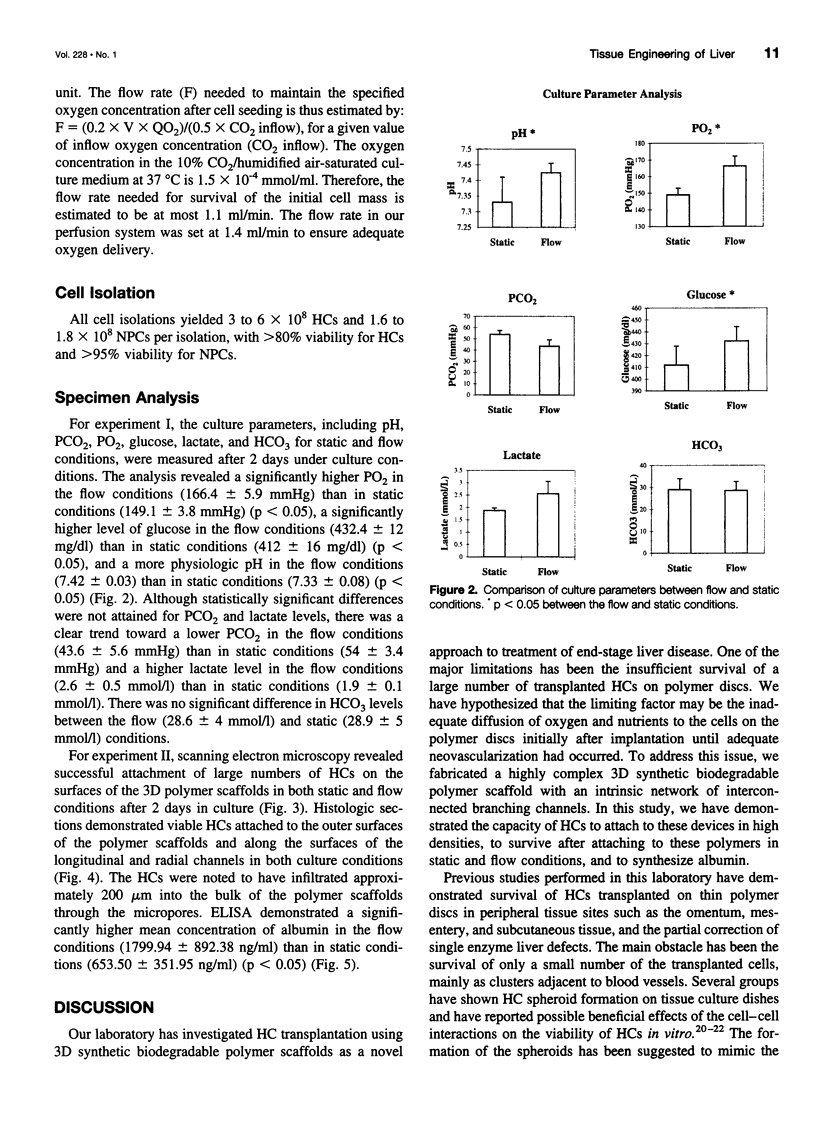
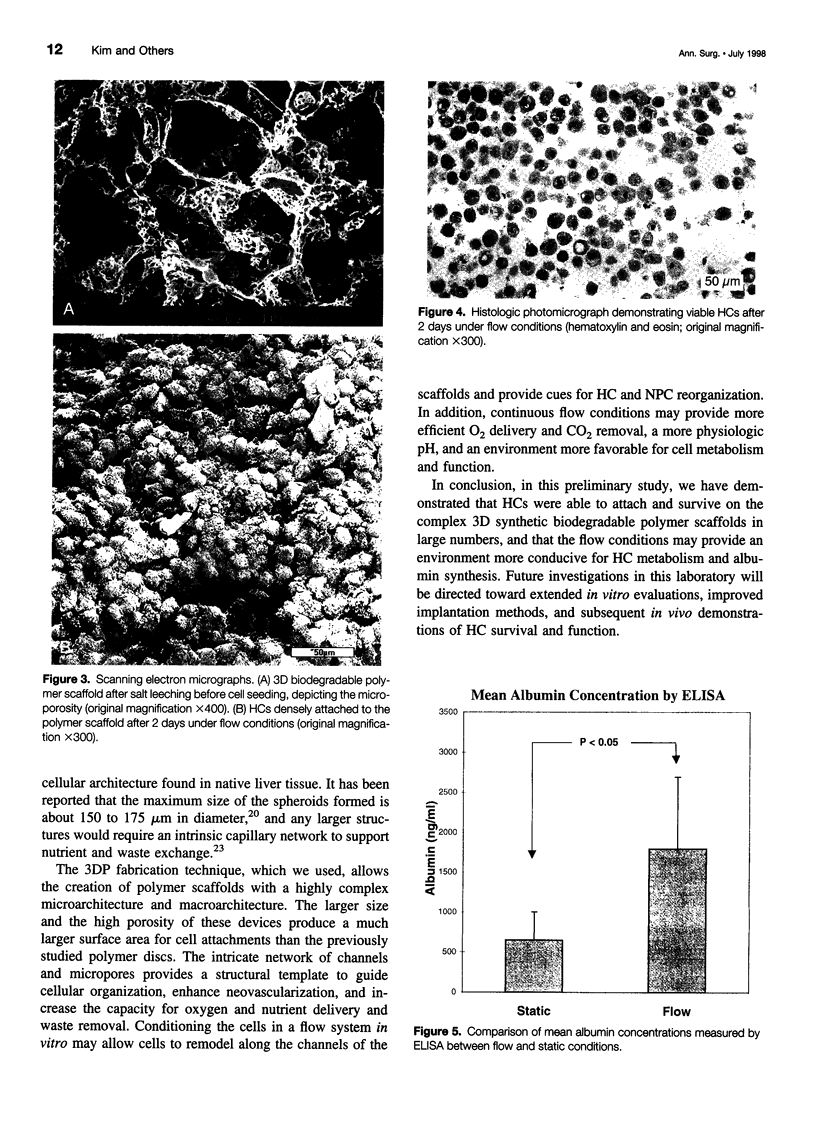

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J., Cima L., Schloo B., Mooney D., Johnson L., Langer R., Vacanti J. P. Studies in rat liver perfusion for optimal harvest of hepatocytes. J Pediatr Surg. 1990 Jan;25(1):140–145. doi: 10.1016/s0022-3468(05)80180-0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973 Oct 1;138(4):745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng L. B., Hsu B. R., Fu S. H., Chuang K. L., Lee W. C., Chen M. F., Chang C. H. Cotransplantation of microencapsulated hepatocytes and islets for acute hepatic failure in rats. Transplant Proc. 1996 Jun;28(3):1859–1860. [PubMed] [Google Scholar]
- Kaufmann P. M., Sano K., Uyama S., Takeda T., Vacanti J. P. Heterotopic hepatocyte transplantation: assessing the impact of hepatotrophic stimulation. Transplant Proc. 1994 Aug;26(4):2240–2241. [PubMed] [Google Scholar]
- Koide N., Shinji T., Tanabe T., Asano K., Kawaguchi M., Sakaguchi K., Koide Y., Mori M., Tsuji T. Continued high albumin production by multicellular spheroids of adult rat hepatocytes formed in the presence of liver-derived proteoglycans. Biochem Biophys Res Commun. 1989 May 30;161(1):385–391. doi: 10.1016/0006-291x(89)91609-4. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Ouellet C., Goyette R., Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985 Sep;101(3):914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Vacanti J. P. Tissue engineering. Science. 1993 May 14;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Mooney D. J., Kaufmann P. M., Sano K., McNamara K. M., Vacanti J. P., Langer R. Transplantation of hepatocytes using porous, biodegradable sponges. Transplant Proc. 1994 Dec;26(6):3425–3426. [PubMed] [Google Scholar]
- Mooney D. J., Park S., Kaufmann P. M., Sano K., McNamara K., Vacanti J. P., Langer R. Biodegradable sponges for hepatocyte transplantation. J Biomed Mater Res. 1995 Aug;29(8):959–965. doi: 10.1002/jbm.820290807. [DOI] [PubMed] [Google Scholar]
- Russell P. S. Selective transplantation. An emerging concept. Ann Surg. 1985 Mar;201(3):255–262. doi: 10.1097/00000658-198503000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shimaoka S., Nakamura T., Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp Cell Res. 1987 Sep;172(1):228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- Takeda T., Kim T. H., Lee S. K., Langer R., Vacanti J. P. Hepatocyte transplantation in biodegradable polymer scaffolds using the Dalmatian dog model of hyperuricosuria. Transplant Proc. 1995 Feb;27(1):635–636. [PubMed] [Google Scholar]
- Tong J. Z., De Lagausie P., Furlan V., Cresteil T., Bernard O., Alvarez F. Long-term culture of adult rat hepatocyte spheroids. Exp Cell Res. 1992 Jun;200(2):326–332. doi: 10.1016/0014-4827(92)90179-c. [DOI] [PubMed] [Google Scholar]
- Vacanti J. P. Beyond transplantation. Third annual Samuel Jason Mixter lecture. Arch Surg. 1988 May;123(5):545–549. doi: 10.1001/archsurg.1988.01400290027003. [DOI] [PubMed] [Google Scholar]
- Vacanti J. P., Morse M. A., Saltzman W. M., Domb A. J., Perez-Atayde A., Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988 Jan;23(1 Pt 2):3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Imazato K., Masumoto A. Growth stimulation of adult rat hepatocytes in a primary culture by soluble factor(s) secreted from nonparenchymal liver cell. Cell Struct Funct. 1989 Apr;14(2):217–229. doi: 10.1247/csf.14.217. [DOI] [PubMed] [Google Scholar]