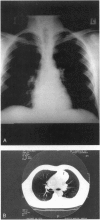
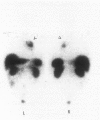
Abstract
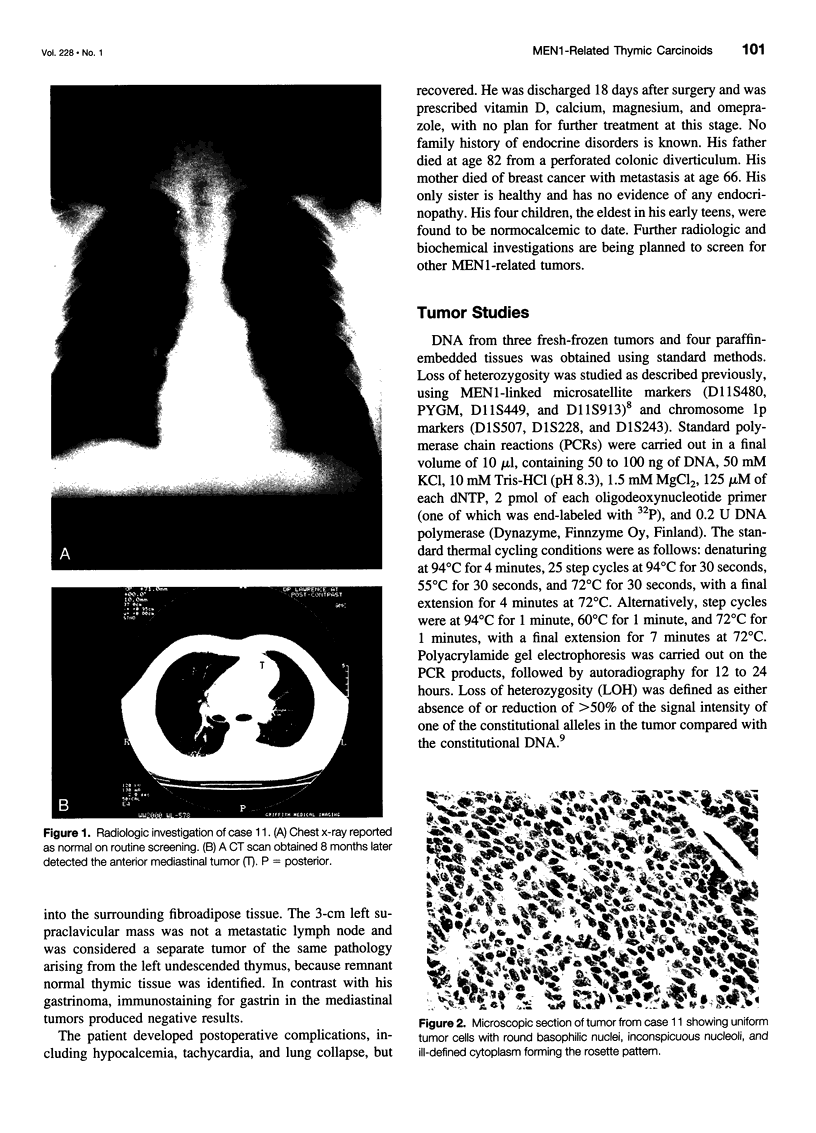
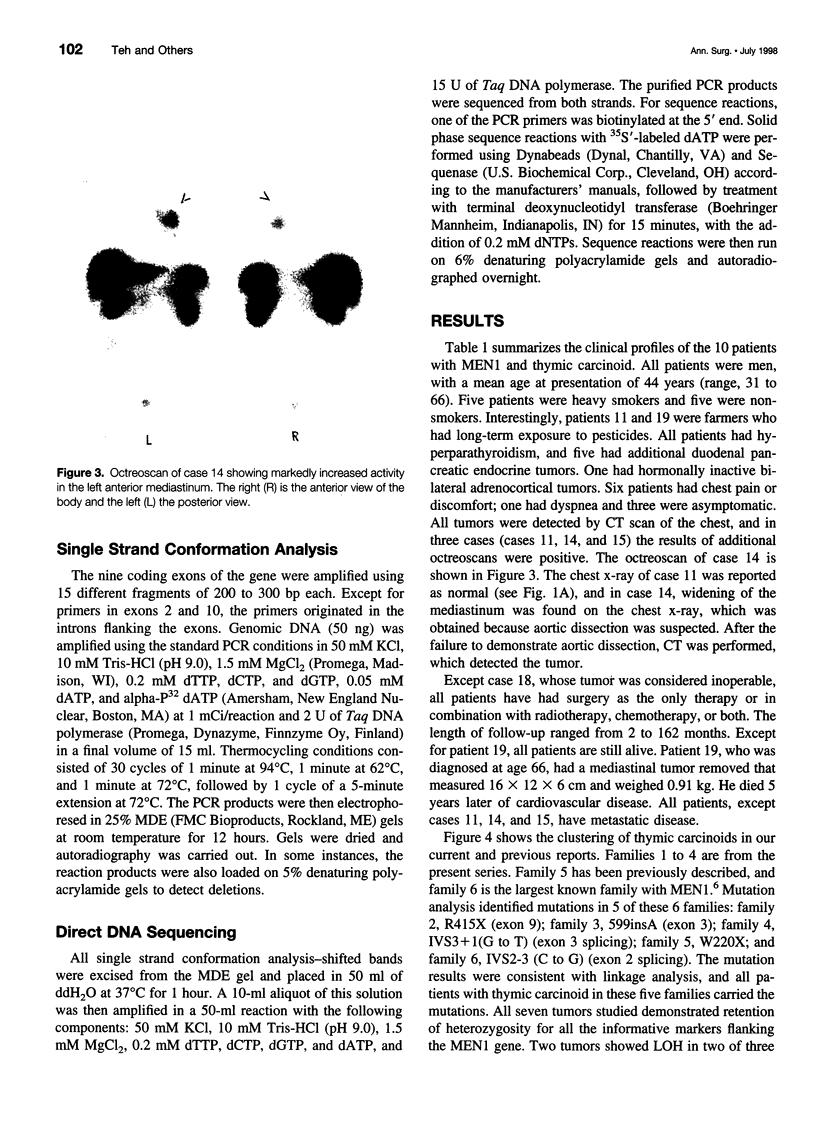
OBJECTIVE: To study the clinical, pathologic, and genetic features of thymic carcinoids in the setting of multiple endocrine neoplasia type 1 (MEN1) and to study means for detection and prevention of this tumor in patients with MEN1. SUMMARY BACKGROUND DATA: Thymic carcinoid is a rare malignancy, with approximately 150 cases reported to date. It may be associated with MEN1 and carries a poor prognosis, with no effective treatment. Its underlying etiology is unknown. METHODS: Ten patients with MEN1 from eight families with anterior mediastinal tumors were included in a case series study at tertiary referring hospitals. Clinicopathologic studies were done on these patients, with a review of the literature. Mutation analysis was performed on the MEN1 gene in families with clusterings of the tumor to look for genotype-phenotype correlation. Loss of heterozygosity was studied in seven cases to look for genetic abnormalities. RESULTS: Histologic studies of all tumors were consistent with the diagnosis of thymic carcinoid. Clustering of this tumor was found in some of the families-three pairs of brothers and three families with first- or second-degree relatives who had thymic carcinoid. All patients described here were men, with a mean age at detection of 44 years (range 31 to 66). Most of the patients had chest pain or were asymptomatic; none had Cushing's or carcinoid syndrome. All tumors were detected by computed tomography (CT) or magnetic resonance imaging (MRI) of the chest. The results of octreoscans performed in three patients were all positive. Histopathologic studies were consistent with the diagnosis of thymic carcinoid and did not stain for ACTH. Mutation analysis of the families with clustering revealed mutations in different exons/introns of the MEN1 gene. Loss of heterozygosity (LOH) studies of seven tumors did not show LOH in the MEN1 region, but two tumors showed LOH in the 1p region. CONCLUSIONS: MEN1-related thymic carcinoids constitute approximately 25% of all cases of thymic carcinoids. In patients with MEN1, this is an insidious tumor not associated with Cushing's or carcinoid syndrome. Local invasion, recurrence, and distant metastasis are common, with no known effective treatment. We propose that CT or MRI of the chest, as well as octreoscanning, should be considered as part of clinical screening in patients with MEN1. We also propose performing prophylactic thymectomy during subtotal or total parathyroidectomy on patients with MEN1 to reduce the risks of thymic carcinoid and recurrence of hyperparathyroidism. Its male predominance, the absence of LOH in the MEN1 region, clustering in close relatives, and the presence of different MEN1 mutations in these families suggest the involvement of modifying genes in addition to the MEN1 gene. A putative tumor suppressor gene in 1p may be involved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandrasekharappa S. C., Guru S. C., Manickam P., Olufemi S. E., Collins F. S., Emmert-Buck M. R., Debelenko L. V., Zhuang Z., Lubensky I. A., Liotta L. A. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997 Apr 18;276(5311):404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- Cryns V. L., Yi S. M., Tahara H., Gaz R. D., Arnold A. Frequent loss of chromosome arm 1p DNA in parathyroid adenomas. Genes Chromosomes Cancer. 1995 May;13(1):9–17. doi: 10.1002/gcc.2870130103. [DOI] [PubMed] [Google Scholar]
- Cupisti K., Dotzenrath C., Simon D., Goretzki P. E., Röher H. D. Chirurgische Therapie neuroendokriner Tumoren des Thymus. Chirurg. 1997 Feb;68(2):136–140. doi: 10.1007/s001040050163. [DOI] [PubMed] [Google Scholar]
- Dobbie Z., Heinimann K., Bishop D. T., Müller H., Scott R. J. Identification of a modifier gene locus on chromosome 1p35-36 in familial adenomatous polyposis. Hum Genet. 1997 May;99(5):653–657. doi: 10.1007/s004390050423. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Harnett P., Bale S. J., Stanger B. Z., Tucker M. A., Housman D. E., Kefford R. F. Loss of alleles from the distal short arm of chromosome 1 occurs late in melanoma tumor progression. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4614–4618. doi: 10.1073/pnas.86.12.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery D. Spindle-cell thymic carcinoid occurring in multiple endocrine neoplasia I: fine-needle aspiration findings in a case. Diagn Cytopathol. 1996 Dec;15(5):439–441. doi: 10.1002/(SICI)1097-0339(199612)15:5<439::AID-DC17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Farley D. R., van Heerden J. A., Grant C. S. Are concomitant surgical procedures acceptable in patients undergoing cervical exploration for primary hyperparathyroidism? Mayo Clin Proc. 1991 Jul;66(7):681–685. doi: 10.1016/s0025-6196(12)62079-5. [DOI] [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud S., Choplin H., Teh B. T., Lespinasse J., Jouvet A., Labat-Moleur F., Lenoir G., Hamon B., Hamon P., Calender A. A large multiple endocrine neoplasia type 1 family with clinical expression suggestive of anticipation. J Clin Endocrinol Metab. 1997 Oct;82(10):3487–3492. doi: 10.1210/jcem.82.10.4052. [DOI] [PubMed] [Google Scholar]
- Khosla S., Patel V. M., Hay I. D., Schaid D. J., Grant C. S., van Heerden J. A., Thibodeau S. N. Loss of heterozygosity suggests multiple genetic alterations in pheochromocytomas and medullary thyroid carcinomas. J Clin Invest. 1991 May;87(5):1691–1699. doi: 10.1172/JCI115186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine G. D., Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol. 1978 Sep;9(5):495–515. doi: 10.1016/s0046-8177(78)80131-2. [DOI] [PubMed] [Google Scholar]
- O'Byrne K. J., Carney D. N. Radiolabelled somatostatin analogue scintigraphy in oncology. Anticancer Drugs. 1996 Jan;7 (Suppl 1):33–44. doi: 10.1097/00001813-199601001-00006. [DOI] [PubMed] [Google Scholar]
- Phelan C. M., Rebbeck T. R., Weber B. L., Devilee P., Ruttledge M. H., Lynch H. T., Lenoir G. M., Stratton M. R., Easton D. F., Ponder B. A. Ovarian cancer risk in BRCA1 carriers is modified by the HRAS1 variable number of tandem repeat (VNTR) locus. Nat Genet. 1996 Mar;12(3):309–311. doi: 10.1038/ng0396-309. [DOI] [PubMed] [Google Scholar]
- Romeo G., McKusick V. A. Phenotypic diversity, allelic series and modifier genes. Nat Genet. 1994 Aug;7(4):451–453. doi: 10.1038/ng0894-451. [DOI] [PubMed] [Google Scholar]
- Rosai J., Higa E., Davie J. Mediastinal endocrine neoplasm in patients with multiple endocrine adenomatosis. A previously unrecognized association. Cancer. 1972 Apr;29(4):1075–1083. doi: 10.1002/1097-0142(197204)29:4<1075::aid-cncr2820290457>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Rosai J., Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer. 1972 Apr;29(4):1061–1074. doi: 10.1002/1097-0142(197204)29:4<1061::aid-cncr2820290456>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Katai M., Itakura Y., Nakajima K., Baba K., Hashizume K. Genetic screening in hereditary multiple endocrine neoplasia type 1: absence of a founder effect among Japanese families. Jpn J Cancer Res. 1996 Sep;87(9):985–994. doi: 10.1111/j.1349-7006.1996.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh B. T., Grimmond S., Shepherd J., Larsson C., Hayward N. Multiple endocrine neoplasia type I: clinical syndrome to molecular genetics. Aust N Z J Surg. 1995 Oct;65(10):708–713. doi: 10.1111/j.1445-2197.1995.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Teh B. T., McArdle J., Chan S. P., Menon J., Hartley L., Pullan P., Ho J., Khir A., Wilkinson S., Larsson C. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Medicine (Baltimore) 1997 Jan;76(1):21–29. doi: 10.1097/00005792-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Trump D., Farren B., Wooding C., Pang J. T., Besser G. M., Buchanan K. D., Edwards C. R., Heath D. A., Jackson C. E., Jansen S. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM. 1996 Sep;89(9):653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- Wick M. R., Scott R. E., Li C. Y., Carney J. A. Carcinoid tumor of the thymus: a clinicopathologic report of seven cases with a review of the literature. Mayo Clin Proc. 1980 Apr;55(4):246–254. [PubMed] [Google Scholar]
- Wilkinson S., Teh B. T., Davey K. R., McArdle J. P., Young M., Shepherd J. J. Cause of death in multiple endocrine neoplasia type 1. Arch Surg. 1993 Jun;128(6):683–690. doi: 10.1001/archsurg.1993.01420180085016. [DOI] [PubMed] [Google Scholar]