Abstract
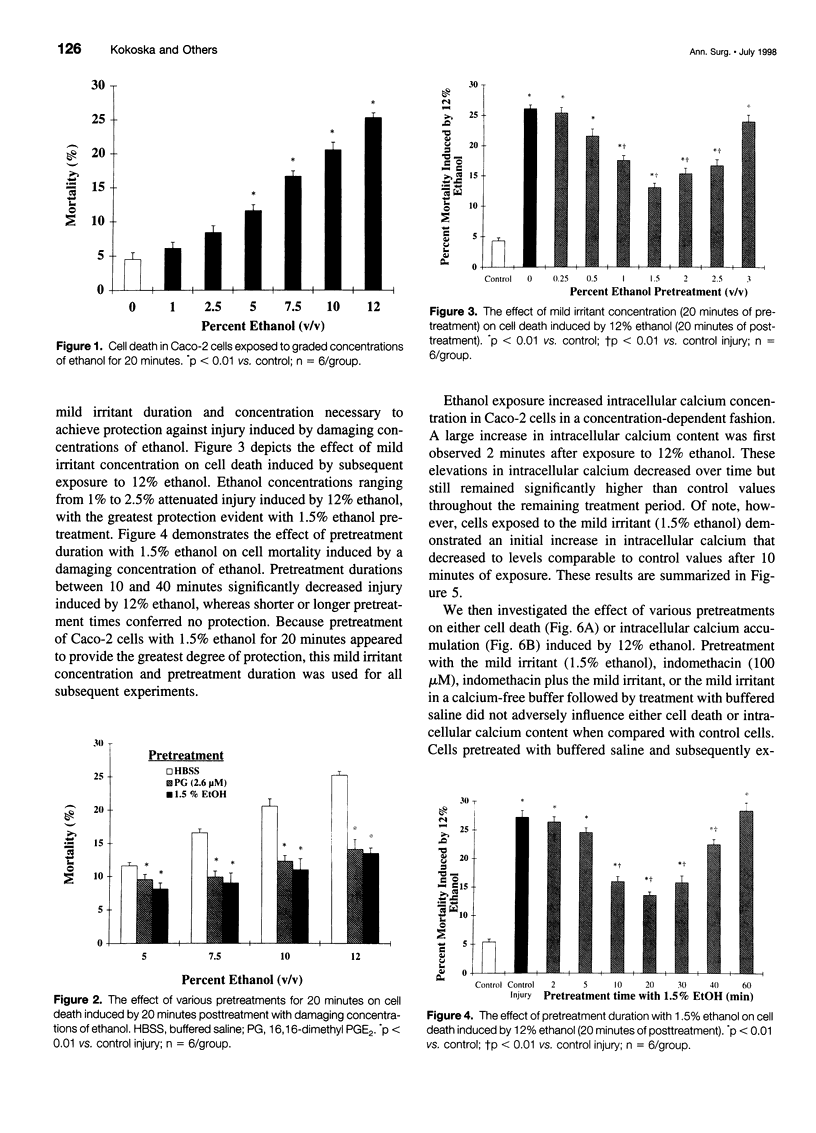
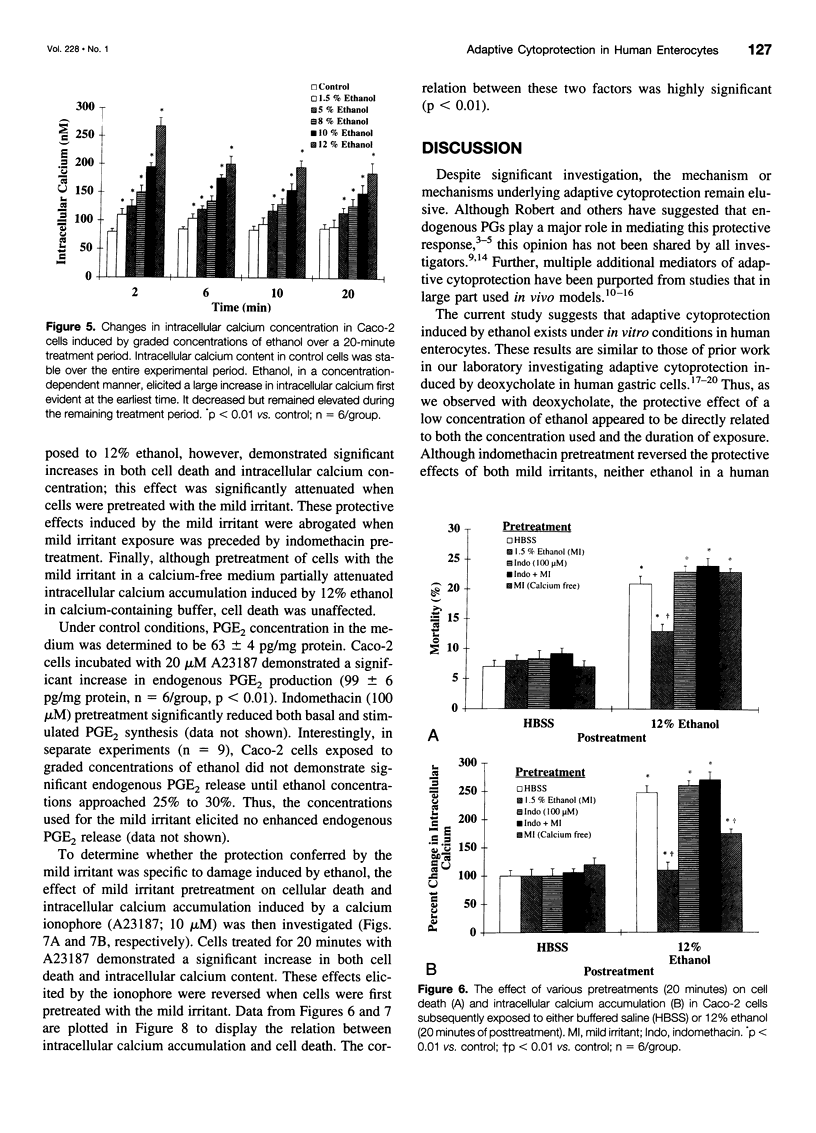
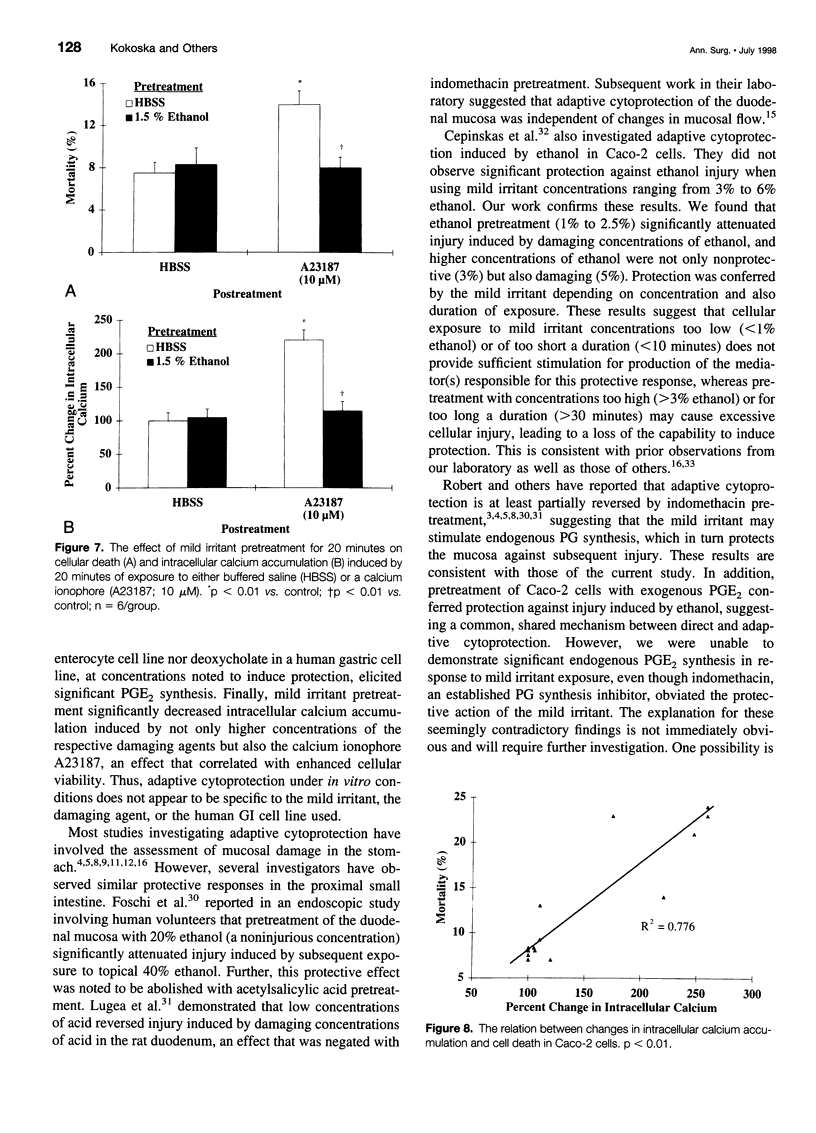
OBJECTIVE: To determine whether adaptive cytoprotection exists in human intestinal cells under in vitro conditions and what role, if any, endogenous prostaglandins or calcium may play in mediating this protective response. SUMMARY BACKGROUND DATA: Adaptive cytoprotection can be defined as that process whereby the administration of a low concentration of a damaging agent, termed a "mild irritant," which by itself is not injurious, can attenuate gastrointestinal mucosal injury subsequently induced by the application of higher concentrations of the same or other necrotizing agents. Despite substantial investigation, the mediator or mediators of adaptive cytoprotection remain poorly understood. METHODS: Postconfluent Caco-2 cells were used in all experiments. Cellular death was quantitated using a dual-component fluorescent assay. Changes in intracellular calcium concentration were quantitated by measuring fluorescent signal changes of the single wavelength calcium indicator (Fluo-3). Finally, prostaglandin E2 release into the media was quantitated by radioimmunoassay. RESULTS: Pretreatment of Caco-2 cells with low concentrations of ethanol (mild irritant) significantly attenuated injury induced by higher damaging concentrations of ethanol. The protection conferred by the mild irritant was directly dependent on both the concentration of the irritant used and the duration of exposure and was abrogated when cells were pretreated with an endogenous prostaglandin inhibitor (indomethacin) or if the mild irritant was administered in calcium-free media. Cells exposed to ethanol had a significant and concentration-dependent increase in intracellular calcium concentration, an effect that was highly related to cellular injury. Pretreatment with a mild irritant significantly decreased intracellular calcium increases induced by not only ethanol but also by a calcium ionophore (A23187). Cells treated with low concentrations of ethanol demonstrated no significant elevation in prostaglandin E2 release. CONCLUSIONS: Adaptive cytoprotection induced by ethanol exists in human colonocytes under in vitro conditions independent of mucosal blood flow, neural innervation, or circulating humoral factors. The authors' data suggest that this response does not require endogenous prostaglandin synthesis but may involve processes whereby intracellular calcium accumulation is prevented.
Full text
PDF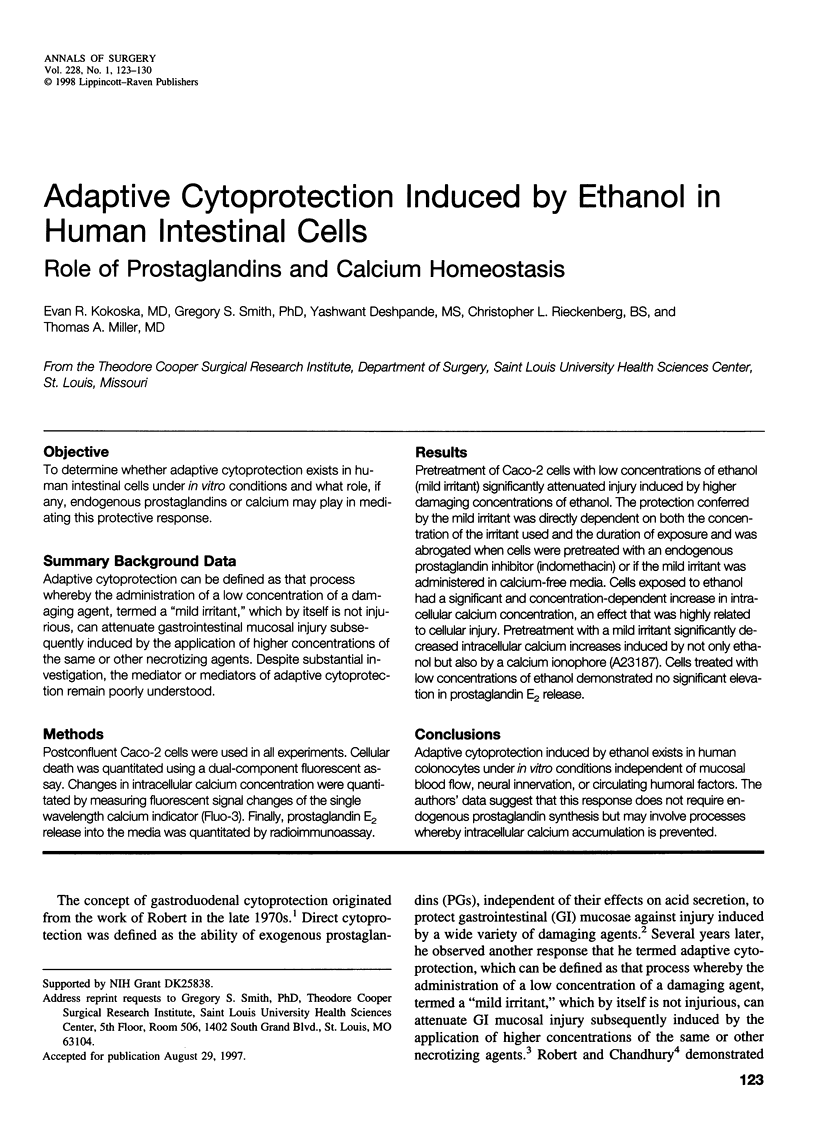




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Nakamura A., Fukuda T., Higuchi K., Nakamura H., Kobayashi K. In vitro adaptive cytoprotection in gastric cells isolated from rat stomach. J Clin Gastroenterol. 1990;12 (Suppl 1):S32–S38. doi: 10.1097/00004836-199001001-00007. [DOI] [PubMed] [Google Scholar]
- Cepinskas G., Specian R. D., Kvietys P. R. Adaptive cytoprotection in the small intestine: role of mucus. Am J Physiol. 1993 May;264(5 Pt 1):G921–G927. doi: 10.1152/ajpgi.1993.264.5.G921. [DOI] [PubMed] [Google Scholar]
- Chaudhury T. K., Robert A. Prevention by mild irritants of gastric necrosis produced in rats by sodium taurocholate. Dig Dis Sci. 1980 Nov;25(11):830–836. doi: 10.1007/BF01338524. [DOI] [PubMed] [Google Scholar]
- Dempsey D. T., Mercer D. W., Deb B., Sauter A., Ritchie W. P., Jr Adaptive cytoprotection of gastric surface epithelial cells against injury by physiologic concentrations of bile acid. Surgery. 1990 Aug;108(2):348–355. [PubMed] [Google Scholar]
- Foschi D., Toti G. L., Del Soldato P., Ferrante F., Galeone M., Rovati V. Adaptive cytoprotection: an endoscopic study in man. Am J Gastroenterol. 1986 Nov;81(11):1035–1037. [PubMed] [Google Scholar]
- Hawkey C. J., Kemp R. T., Walt R. P., Bhaskar N. K., Davies J., Filipowicz B. Evidence that adaptive cytoprotection in rats is not mediated by prostaglandins. Gastroenterology. 1988 Apr;94(4):948–954. doi: 10.1016/0016-5085(88)90552-5. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Jacobson E. D. Direct and adaptive cytoprotection. Dig Dis Sci. 1986 Feb;31(2 Suppl):28S–31S. doi: 10.1007/BF01309319. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Kao J. P. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- Kauffman G. L., Jr Putative mediator(s) of adaptive cytoprotection? Prostaglandins. 1991 Mar;41(3):201–205. doi: 10.1016/0090-6980(91)90040-m. [DOI] [PubMed] [Google Scholar]
- Ko J. K., Cho C. H., Ogle C. W. A correlative study on the mechanism of adaptive cytoprotection against ethanol-induced gastric lesion formation in rats. J Gastroenterol Hepatol. 1994 Sep-Oct;9(5):492–500. doi: 10.1111/j.1440-1746.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Kokoska E. R., Smith G. S., Rieckenberg C. L., Deshpande Y., Banan A., Miller T. A. Adaptive cytoprotection against deoxycholate-induced injury in human gastric cells in vitro: is there a role for endogenous prostaglandins? Dig Dis Sci. 1998 Apr;43(4):806–815. doi: 10.1023/a:1018826416864. [DOI] [PubMed] [Google Scholar]
- Kristensen S. R. Mechanisms of cell damage and enzyme release. Dan Med Bull. 1994 Sep;41(4):423–433. [PubMed] [Google Scholar]
- Lugea A., Salas A., Guarner F., Azpiroz F., Malagelada J. R. Duodenal mucosal resistance to intraluminal acid in the rat: role of adaptive cytoprotection. Gastroenterology. 1992 Apr;102(4 Pt 1):1129–1135. [PubMed] [Google Scholar]
- Lugea A., Salas A., Guarner F., Malagelada J. R. Adaptive cytoprotection of the rat duodenum is not dependent on nitric oxide-induced changes in blood flow. Am J Physiol. 1993 May;264(5 Pt 1):G994–1000. doi: 10.1152/ajpgi.1993.264.5.G994. [DOI] [PubMed] [Google Scholar]
- Miller T. A. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983 Nov;245(5 Pt 1):G601–G623. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- Mutoh H., Ota S., Hiraishi H., Ivey K. J., Terano A., Sugimoto T. Adaptive cytoprotection in cultured rat gastric mucus-producing cells. Role of mucus and prostaglandin synthesis. Dig Dis Sci. 1995 Apr;40(4):872–878. doi: 10.1007/BF02064994. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979 Oct;77(4 Pt 1):761–767. [PubMed] [Google Scholar]
- Robert A., Nezamis J. E., Lancaster C., Hanchar A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979 Sep;77(3):433–443. [PubMed] [Google Scholar]
- Schepp W., Steffen B., Schusdziarra V., Classen M. Calcium, calmodulin, and cyclic adenosine monophosphate modulate prostaglandin E2 release from isolated human gastric mucosal cells. J Clin Endocrinol Metab. 1986 Oct;63(4):886–891. doi: 10.1210/jcem-63-4-886. [DOI] [PubMed] [Google Scholar]
- Schmidt K. L., Smith G. S., Miller T. A. Microscopic correlates of adaptive cytoprotection in an ethanol injury model. Histol Histopathol. 1989 Jan;4(1):105–115. [PubMed] [Google Scholar]
- Sei Y., Arora P. K. Quantitative analysis of calcium (Ca2+) mobilization after stimulation with mitogens or anti-CD3 antibodies. Simultaneous fluo-3 and immunofluorescence flow cytometry. J Immunol Methods. 1991 Mar 21;137(2):237–244. doi: 10.1016/0022-1759(91)90029-f. [DOI] [PubMed] [Google Scholar]
- Smith G. S., Myers S. I., Bartula L. L., Miller T. A. Adaptive cytoprotection against alcohol injury in the rat stomach is not due to increased prostanoid synthesis. Prostaglandins. 1991 Mar;41(3):207–223. doi: 10.1016/0090-6980(91)90041-d. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Soper B. D. Effect of extracellular Ca2+ on indomethacin-induced injury to rabbit dispersed gastric mucosal cells. Can J Physiol Pharmacol. 1994 Jan;72(1):63–69. doi: 10.1139/y94-011. [DOI] [PubMed] [Google Scholar]
- Tripp M. A., Tepperman B. L. Role of calcium in nitric oxide-mediated injury to rat gastric mucosal cells. Gastroenterology. 1996 Jul;111(1):65–72. doi: 10.1053/gast.1996.v111.pm8698226. [DOI] [PubMed] [Google Scholar]


