Abstract
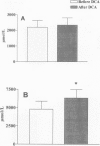
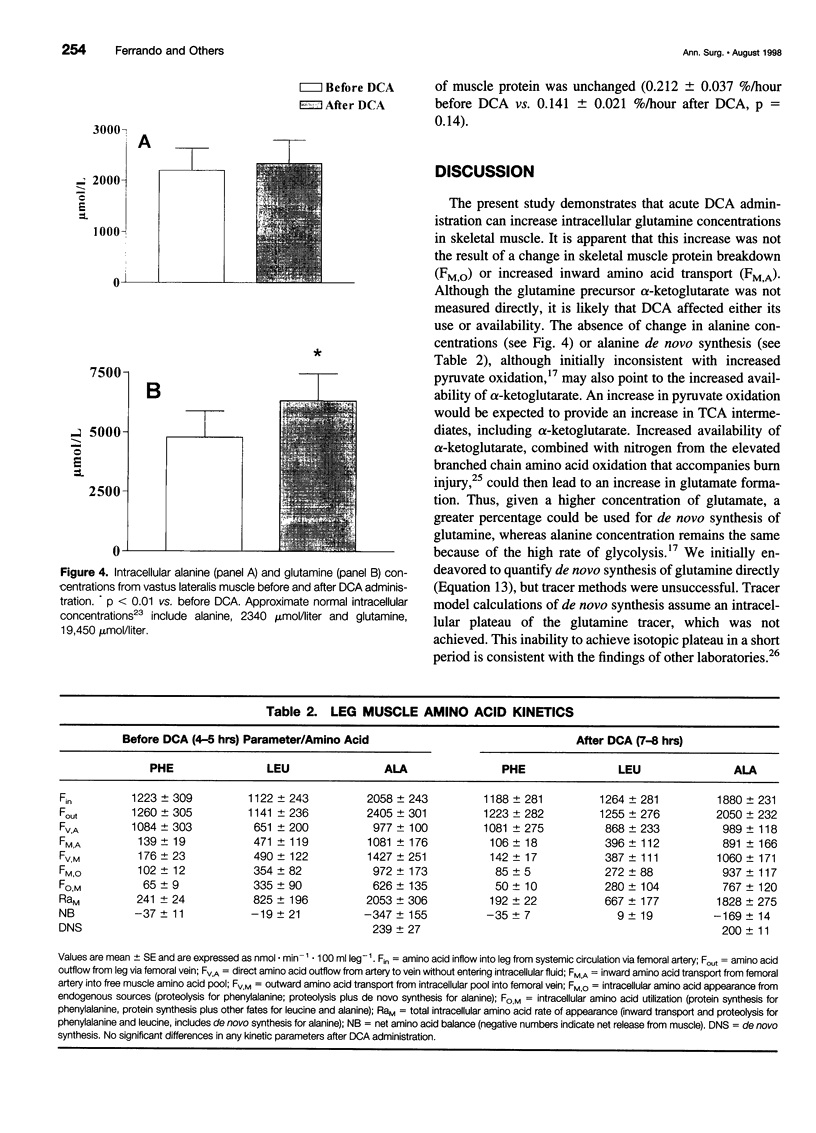
OBJECTIVE: To investigate the hypothesis that the stimulation of pyruvate oxidation by dichloroacetate (DCA) administration would increase the level of intramuscular glutamine in severely burned patients. SUMMARY BACKGROUND DATA: The level of intramuscular glutamine decreases in response to severe injury, and the rate of intramuscular glycolysis and pyruvate oxidation is elevated. Intramuscular glutamine concentrations have been correlated to muscle protein synthesis. METHODS: Six studies were conducted on five patients with burns >40% total body surface area. Patients were studied in the fed state during an 8-hour stable isotope infusion. After 5 hours, DCA (30 mg/kg) was administered for 30 minutes. RESULTS: Analysis of muscle biopsy samples taken at 5 and 8 hours of the study revealed a 32% increase in intracellular glutamine levels after DCA administration. Increased intracellular glutamine concentrations did not affect skeletal muscle protein synthesis as determined by a three-pool arteriovenous model or by the direct incorporation of isotope into skeletal muscle protein. DCA administration resulted in a decrease in plasma lactate but no change in alanine de novo synthesis or intracellular concentration. CONCLUSIONS: These results suggest that acute DCA administration can increase intramuscular glutamine concentration, but that this acute elevation does not affect muscle protein metabolism.
Full text
PDF
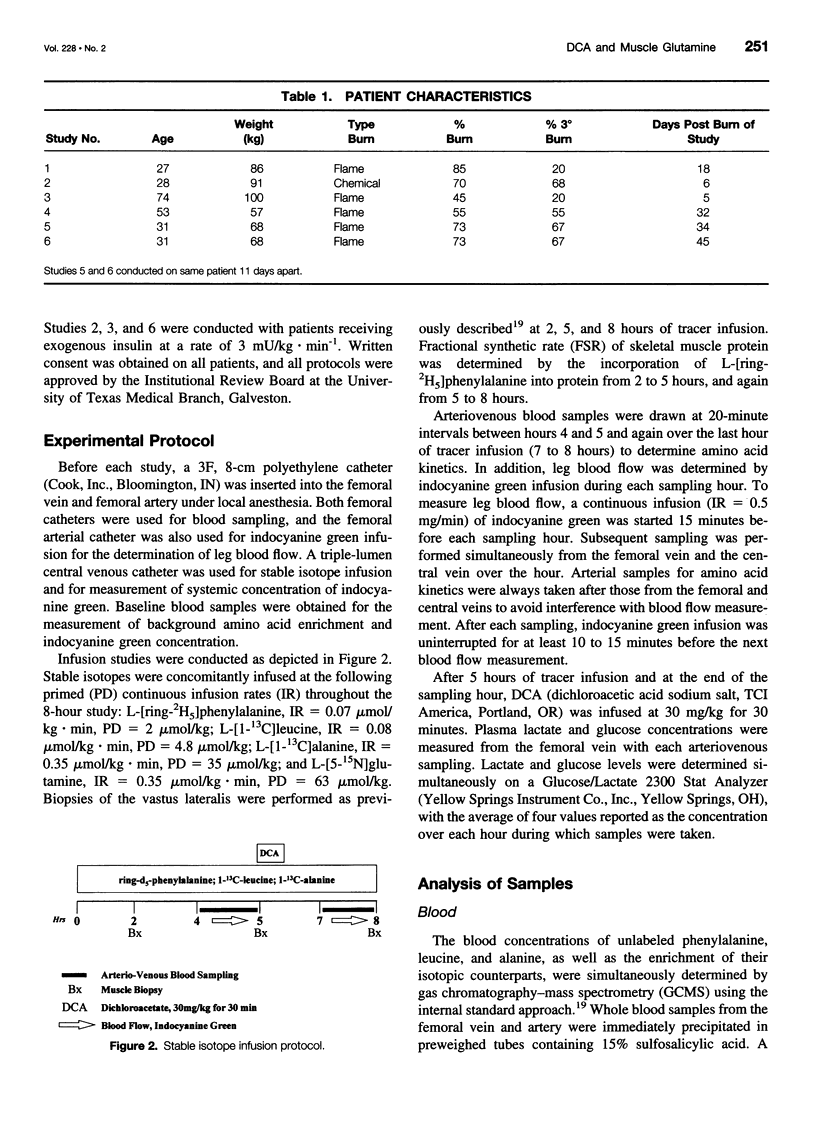

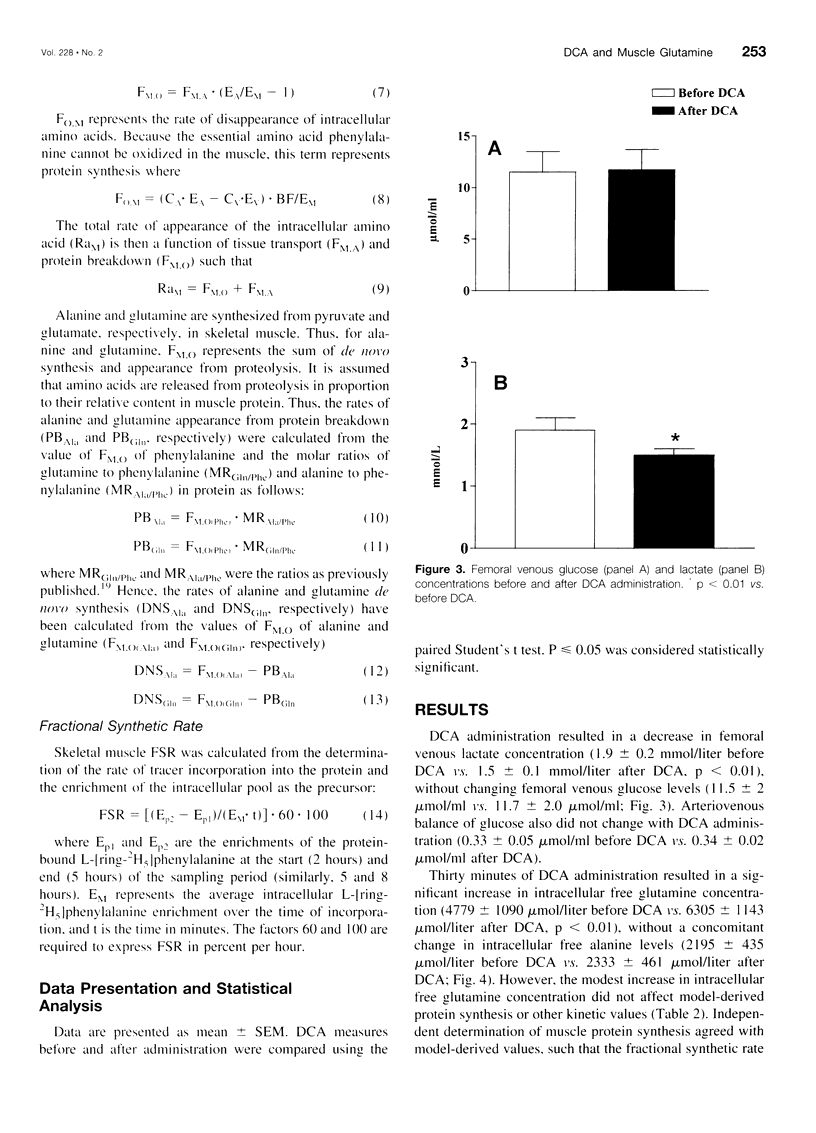


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström J., Fürst P., Norée L. O., Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974 Jun;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- Biolo G., Chinkes D., Zhang X. J., Wolfe R. R. Harry M. Vars Research Award. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enteral Nutr. 1992 Jul-Aug;16(4):305–315. doi: 10.1177/0148607192016004305. [DOI] [PubMed] [Google Scholar]
- Biolo G., Declan Fleming R. Y., Wolfe R. R. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995 Feb;95(2):811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G., Fleming R. Y., Maggi S. P., Wolfe R. R. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995 Jan;268(1 Pt 1):E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- Biolo G., Tipton K. D., Klein S., Wolfe R. R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997 Jul;273(1 Pt 1):E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Brown J., Gore D. C., Lee R. Dichloroacetate inhibits peripheral efflux of pyruvate and alanine during hormonally simulated catabolic stress. J Surg Res. 1993 Jun;54(6):592–596. doi: 10.1006/jsre.1993.1090. [DOI] [PubMed] [Google Scholar]
- Caldwell M. D. Local glutamine metabolism in wounds and inflammation. Metabolism. 1989 Aug;38(8 Suppl 1):34–39. doi: 10.1016/0026-0495(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Ferrando A. A., Lane H. W., Stuart C. A., Davis-Street J., Wolfe R. R. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996 Apr;270(4 Pt 1):E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Gamrin L., Essén P., Forsberg A. M., Hultman E., Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy-rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Crit Care Med. 1996 Apr;24(4):575–583. doi: 10.1097/00003246-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Gore D. C., Jahoor F. Glutamine kinetics in burn patients. Comparison with hormonally induced stress in volunteers. Arch Surg. 1994 Dec;129(12):1318–1323. doi: 10.1001/archsurg.1994.01420360108015. [DOI] [PubMed] [Google Scholar]
- Gore D. C., Jahoor F., Hibbert J., DeMaria E. J. Except for alanine, muscle protein catabolism is not influenced by alterations in glucose metabolism during sepsis. Arch Surg. 1995 Nov;130(11):1171–1177. doi: 10.1001/archsurg.1995.01430110029006. [DOI] [PubMed] [Google Scholar]
- Hammarqvist F., Wernerman J., Ali R., von der Decken A., Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989 Apr;209(4):455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarqvist F., Wernerman J., von der Decken A., Vinnars E. Alpha-ketoglutarate preserves protein synthesis and free glutamine in skeletal muscle after surgery. Surgery. 1991 Jan;109(1):28–36. [PubMed] [Google Scholar]
- Hankard R. G., Darmaun D., Sager B. K., D'Amore D., Parsons W. R., Haymond M. Response of glutamine metabolism to exogenous glutamine in humans. Am J Physiol. 1995 Oct;269(4 Pt 1):E663–E670. doi: 10.1152/ajpendo.1995.269.4.E663. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Desai M., Herndon D. N., Wolfe R. R. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988 Apr;37(4):330–337. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Kapadia C. R., Colpoys M. F., Jiang Z. M., Johnson D. J., Smith R. J., Wilmore D. W. Maintenance of skeletal muscle intracellular glutamine during standard surgical trauma. JPEN J Parenter Enteral Nutr. 1985 Sep-Oct;9(5):583–589. doi: 10.1177/0148607185009005583. [DOI] [PubMed] [Google Scholar]
- Lacey J. M., Wilmore D. W. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990 Aug;48(8):297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Marano M. A., Campbell R. G. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol. 1993 Jun;264(6 Pt 1):E848–E854. doi: 10.1152/ajpendo.1993.264.6.E848. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle C. K., Ogle J. D., Mao J. X., Simon J., Noel J. G., Li B. G., Alexander J. W. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN J Parenter Enteral Nutr. 1994 Mar-Apr;18(2):128–133. doi: 10.1177/0148607194018002128. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Burrin D. G., Jahoor F., Wykes L., Henry J., Frazer E. M. Enteral glutamate is almost completely metabolized in first pass by the gastrointestinal tract of infant pigs. Am J Physiol. 1996 Mar;270(3 Pt 1):E413–E418. doi: 10.1152/ajpendo.1996.270.3.E413. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Hundal H. S., Babij P., MacLennan P., Taylor P. M., Watt P. W., Jepson M. M., Millward D. J. Characteristics of a glutamine carrier in skeletal muscle have important consequences for nitrogen loss in injury, infection, and chronic disease. Lancet. 1986 Nov 1;2(8514):1008–1012. doi: 10.1016/s0140-6736(86)92617-6. [DOI] [PubMed] [Google Scholar]
- Wernerman J., Hammarqvist F., Vinnars E. Alpha-ketoglutarate and postoperative muscle catabolism. Lancet. 1990 Mar 24;335(8691):701–703. doi: 10.1016/0140-6736(90)90811-i. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Goodenough R. D., Burke J. F., Wolfe M. H. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg. 1983 Feb;197(2):163–171. doi: 10.1097/00000658-198302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R. Herman Award Lecture, 1996: relation of metabolic studies to clinical nutrition--the example of burn injury. Am J Clin Nutr. 1996 Nov;64(5):800–808. doi: 10.1093/ajcn/64.5.800. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Jahoor F., Herndon D. N., Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991 Jul;110(1):54–67. [PubMed] [Google Scholar]