Abstract
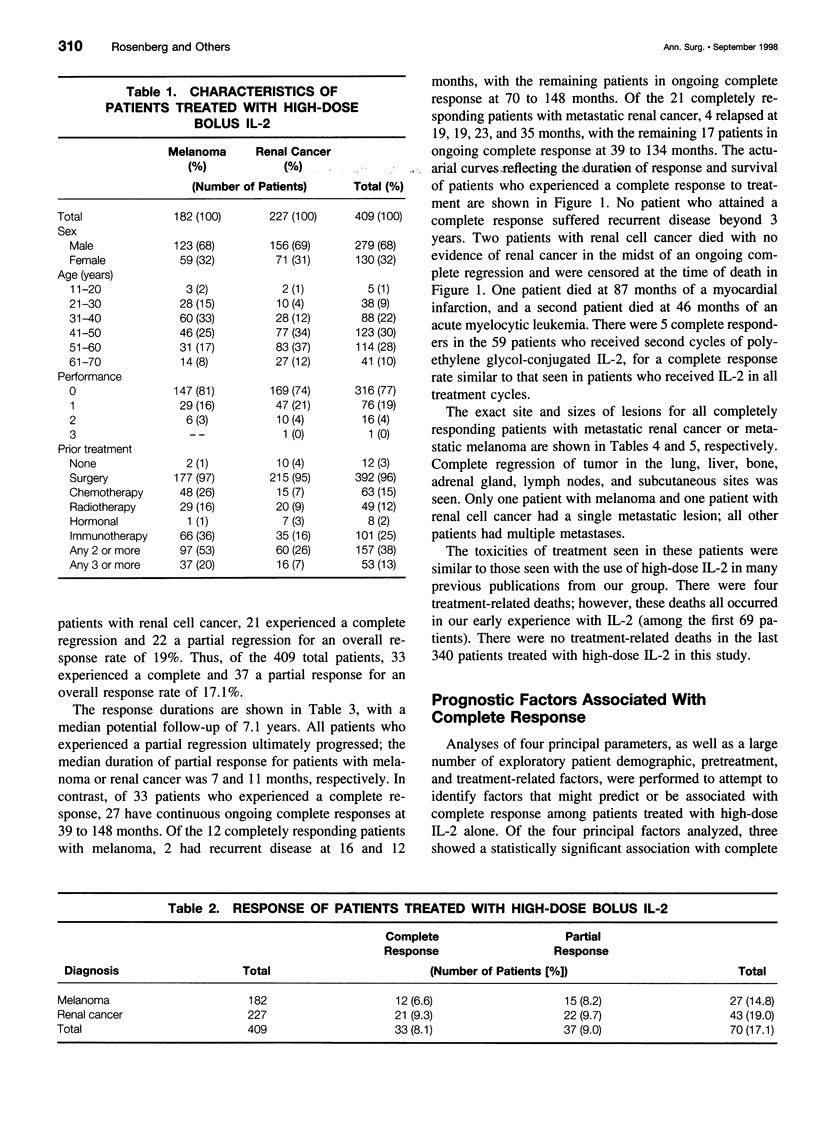
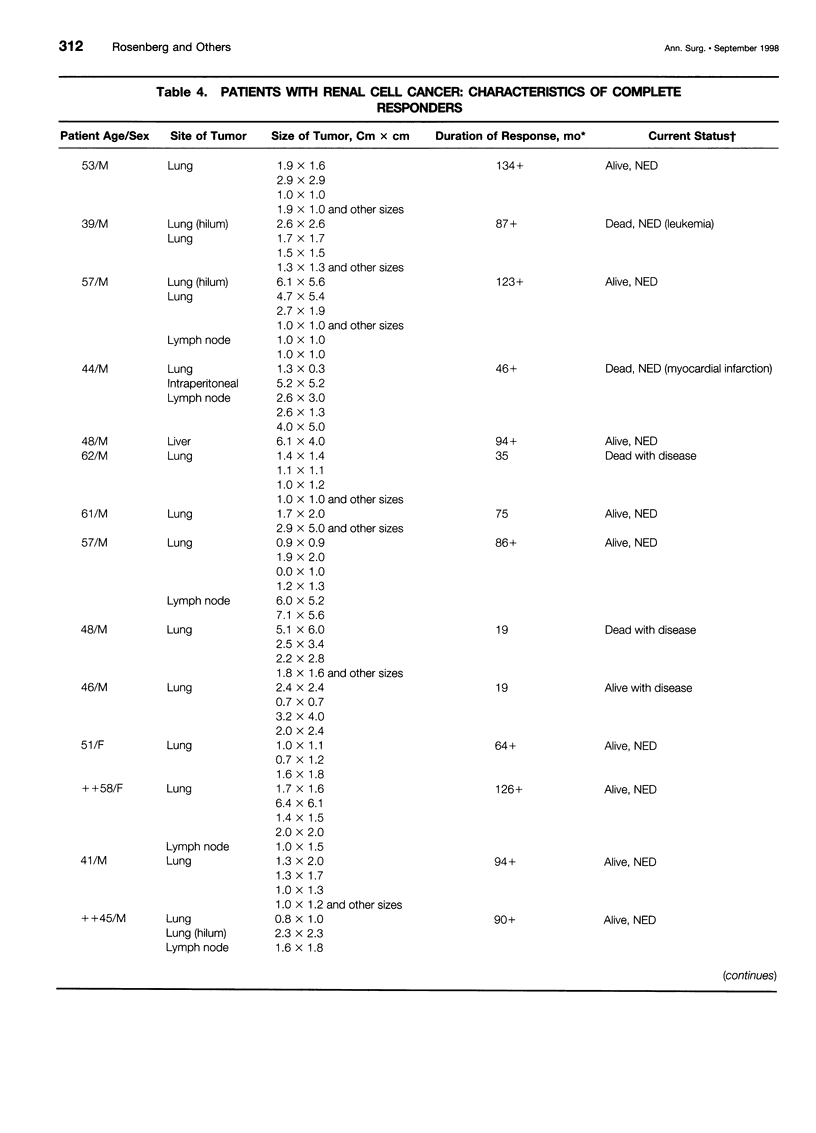
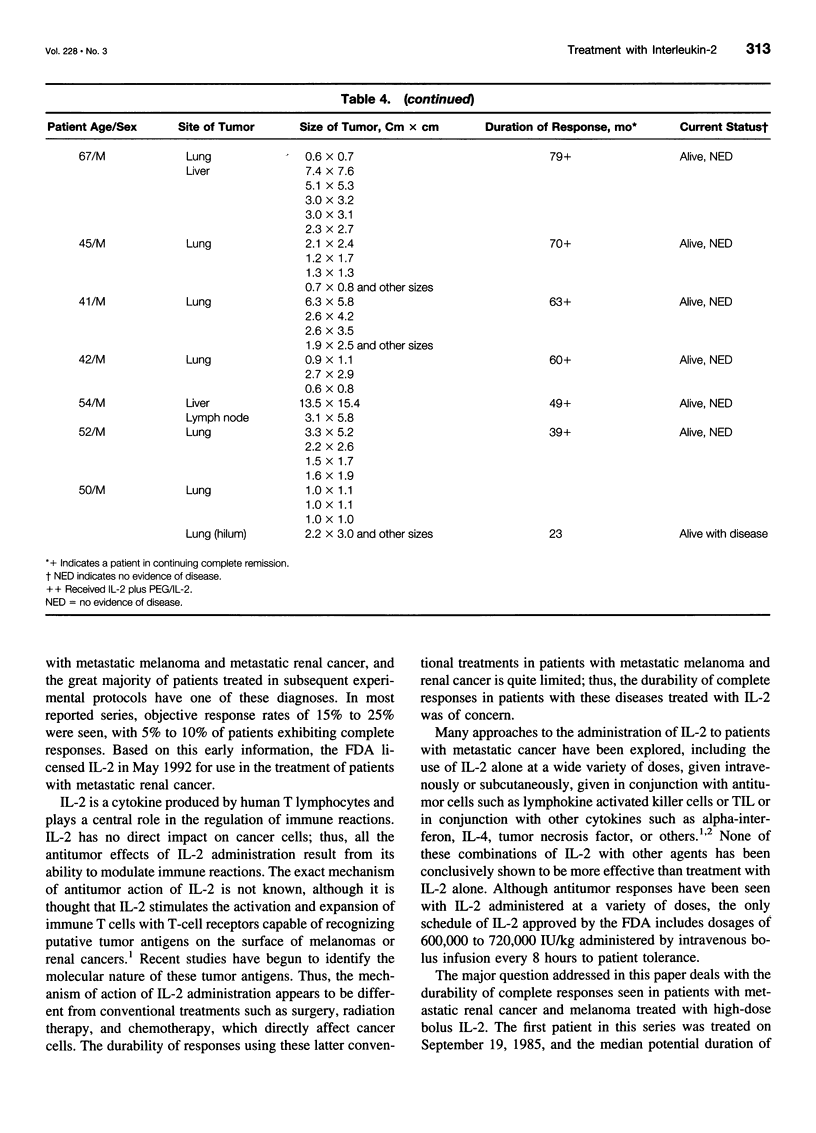
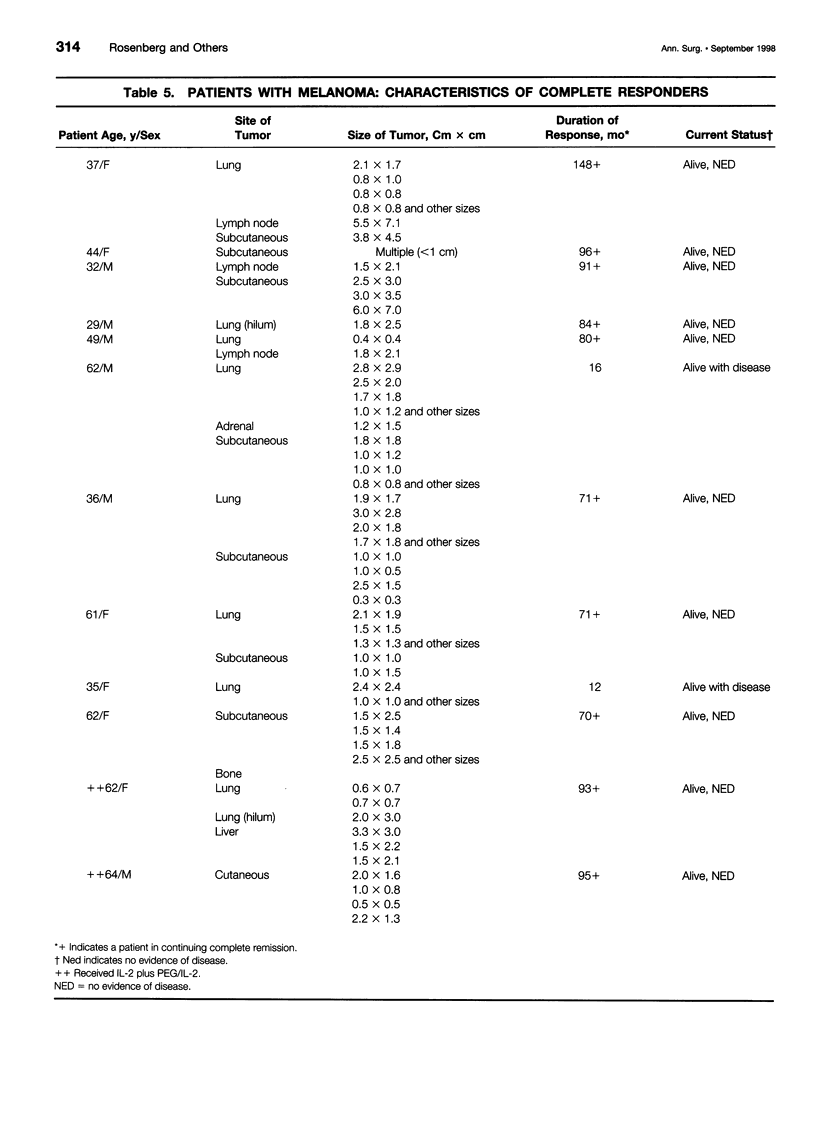
OBJECTIVE: To determine the durability of complete responses in patients with metastatic melanoma or renal cancer treated with high-dose bolus interleukin-2 (IL-2) as well as the factors associated with the development of a complete response and the antigens mediating clinical responses. METHODS: A consecutive series of 409 patients with either metastatic melanoma or renal cancer who were treated with high-dose bolus IL-2 in the Surgery Branch, National Cancer Institute, between September 1985 and November 1996 have been analyzed with a median potential follow-up of 7.1 years. All patients were treated with 720,000 IU/kg administered by 15-minute intravenous infusions every 8 hours for up to 5 days as clinically tolerated per cycle. Two cycles constituted a treatment course. Tumor-infiltrating lymphocytes (TIL) from melanoma patients were used to clone the genes encoding the tumor antigens responsible for clinical responsiveness. RESULTS: Thirty-three of 409 (8.1%) patients treated with high-dose bolus IL-2 achieved a complete response and 37 (9%) achieved a partial response. Complete regression was seen in 6.6% and 9.3% of patients with metastatic melanoma and renal cancer, respectively. Twenty-seven of these 33 completely responding patients (82%) remain in ongoing continuous complete response from 39 to more than 148 months from the onset of treatment. Tumor regressions were seen at virtually all organ sites. The absence of prior treatment with immunotherapy, the total dose of IL-2 administered, and the maximal rebound lymphocytosis after cessation of IL-2 correlated with achieving a complete response. Expression cloning techniques have identified a series of tumor antigens that are recognized by TIL grown from resected melanomas. These antigens are mainly melanoma/ melanocyte differentiation antigens, although mutated intracellular proteins can also serve as antigens. CONCLUSIONS: Treatment with high-dose bolus IL-2 mediates complete cancer regression in approximately 8% of patients with metastatic renal cancer and melanoma. The great majority of these patients will enter durable complete regressions and appear to be cured of their metastatic cancer. Thus, immunotherapy with high-dose bolus IL-2 should be considered as initial therapy for appropriately selected patients with metastatic melanoma and renal cell cancer. Identification of the tumor antigens mediating clinical response is opening new therapeutic possibilities for cancer treatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fyfe G. A., Fisher R. I., Rosenberg S. A., Sznol M., Parkinson D. R., Louie A. C. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996 Aug;14(8):2410–2411. doi: 10.1200/JCO.1996.14.8.2410. [DOI] [PubMed] [Google Scholar]
- Fyfe G., Fisher R. I., Rosenberg S. A., Sznol M., Parkinson D. R., Louie A. C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995 Mar;13(3):688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- Kang X., Kawakami Y., el-Gamil M., Wang R., Sakaguchi K., Yannelli J. R., Appella E., Rosenberg S. A., Robbins P. F. Identification of a tyrosinase epitope recognized by HLA-A24-restricted, tumor-infiltrating lymphocytes. J Immunol. 1995 Aug 1;155(3):1343–1348. [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Rivoltini L., Topalian S. L., Miki T., Rosenberg S. A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Sakaguchi K., Appella E., Yannelli J. R., Adema G. J., Miki T., Rosenberg S. A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Jennings C., Sakaguchi K., Kang X., Southwood S., Robbins P. F., Sette A., Appella E., Rosenberg S. A. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995 Apr 15;154(8):3961–3968. [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Sakaguchi K., Robbins P. F., Rivoltini L., Yannelli J. R., Appella E., Rosenberg S. A. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994 Jul 1;180(1):347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Robbins P. F., El-Gamil M., Li Y. F., Kawakami Y., Loftus D., Appella E., Rosenberg S. A. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996 Mar 1;183(3):1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Kawakami Y., Stevens E., Yannelli J. R., Rosenberg S. A. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994 Jun 15;54(12):3124–3126. [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Li Y. F., Topalian S. L., Rivoltini L., Sakaguchi K., Appella E., Kawakami Y., Rosenberg S. A. Cloning of a new gene encoding an antigen recognized by melanoma-specific HLA-A24-restricted tumor-infiltrating lymphocytes. J Immunol. 1995 Jun 1;154(11):5944–5950. [PubMed] [Google Scholar]
- Rosenberg S. A. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997 Apr;18(4):175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., White D. E. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996 Jan;19(1):81–84. [PubMed] [Google Scholar]
- Rosenberg S. A., Yang J. C., Topalian S. L., Schwartzentruber D. J., Weber J. S., Parkinson D. R., Seipp C. A., Einhorn J. H., White D. E. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994 Mar 23;271(12):907–913. [PubMed] [Google Scholar]
- Rosenberg S. A., Yannelli J. R., Yang J. C., Topalian S. L., Schwartzentruber D. J., Weber J. S., Parkinson D. R., Seipp C. A., Einhorn J. H., White D. E. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994 Aug 3;86(15):1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Rosenberg S. A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989 May 15;142(10):3714–3725. [PubMed] [Google Scholar]
- Wang R. F., Parkhurst M. R., Kawakami Y., Robbins P. F., Rosenberg S. A. Utilization of an alternative open reading frame of a normal gene in generating a novel human cancer antigen. J Exp Med. 1996 Mar 1;183(3):1131–1140. doi: 10.1084/jem.183.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. F., Robbins P. F., Kawakami Y., Kang X. Q., Rosenberg S. A. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995 Feb 1;181(2):799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. C., Topalian S. L., Schwartzentruber D. J., Parkinson D. R., Marincola F. M., Weber J. S., Seipp C. A., White D. E., Rosenberg S. A. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer. 1995 Aug 15;76(4):687–694. doi: 10.1002/1097-0142(19950815)76:4<687::aid-cncr2820760424>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]


