Summary
Prenylation is the addition of prenyl groups to peptide chains or metabolites via the condensation of geranyl- or isopentenyl-diphosphate moieties by geranyltranstransferases. Although this process is extensively studied in eukaryotes, little is known about the influence of prenylation in prokaryotic species. To explore the role of this modification in bacteria, we generated a mutation in the geranyltranstransferase (IspA) of Staphylococcus aureus. Quite strikingly, the ispA mutant completely lacked pigment and exhibited a previously undescribed small colony variant-like phenotype. Further pleiotropic defects in cellular behavior were noted, including impaired growth, decreased ATP production, increased sensitivity to oxidative stress, increased resistance to aminoglycosides and cationic antimicrobial peptides, and decreased resistance to cell wall-targeting antibiotics. These latter effects appear to result from differences in envelope composition as ispA mutants have highly diffuse cell walls (particularly at the septum), marked alterations in fatty acid composition and increased membrane fluidity. Taken together, these data present an important characterization of prokaryotic prenylation and demonstrate that this process is central to a wealth of pathways involved in mediating cellular homeostasis in S. aureus.
Introduction
Staphylococcus aureus is a leading health concern due to its ability to cause a multitude of diseases, which lead to millions of hospitalizations yearly. The success of the pathogen can be attributed to its many virulence determinants and their coordinated regulation, which allow it to infect the host and evade immune responses. In addition to regulation by modulating gene transcription, regulation can also be achieved by post-translational modifications (PTMs), such as phosphorylation, lipidation, acetylation, methylation and glycosylation (Cain et al., 2014).
Prenylation is used by eukaryotes as a key PTM; however, this process has not been well studied in prokaryotes. Prenylation is the addition of a prenyl (3-methyl-2-butenyl), or isoprenoid, group to a substrate. Mechanistically, prenylation of eukaryotic proteins is achieved as a series of steps (Zhang and Casey, 1996). Firstly, the protein to be post-translationally modified is recognized by its conserved ‘CAAX’ C-terminal motif (‘C’ refers to cysteine, ‘A’ any aliphatic acid and ‘X’ any amino acid). The cysteine is prenylated by the addition of either a 15-carbon moiety through a farnesyl transferase (FTase) or a 20-carbon moiety via a geranylgeranyl transferase I. This modified C-terminus can then be processed by a CAAX protease, wherein the -AAX tripeptide is removed. Once this tripeptide is released from the protein, the new C-terminus is methylated by an isoprenylcysteine carboxyl methyltransferase. In addition to studies of prenylation as a PTM, prenylation of a variety of eukaryotic metabolites including sterols, quinones and chlorophyll has also been demonstrated (Espenshade and Hughes, 2007). Collectively, the prenylation of both metabolites and proteins is a key process required to maintain homeostasis in eukaryotic cells.
Although a number of prenylated targets have been discovered in eukaryotes, the identification of prenylated substrates in prokaryotes has been limited. In bacteria, a lone protein has been identified as being prenylated: ComX, a quorum sensing pheromone, is prenylated in species of Bacilli (Okada et al., 2008; Tsuji et al., 2011). Specifically, this processed peptide receives either a farnesyl or geranylgeranyl modification at a C-terminally located tryptophan (rather than the cysteine observed in eukaryotes) in a number of species (Okada, 2011). Importantly, although several ComX peptides have been identified as undergoing prenylation at tryptophan residues, a consensus sequence for this modification has not been determined, unlike the CAAX sequence seen in eukaryotic proteins (Tsuji et al., 2012). Additionally, it has been shown that effector proteins AnkB in Legionella pneumophila and SifA in Salmonella enterica can be prenylated by host machinery upon injection into eukaryotic cells (Reinicke et al., 2005; Price et al., 2010). More recently, it has been found that SelU-mediated geranylation of RNA occurs in several Gram-negative bacteria (Dumelin et al., 2012).
In contrast to protein modification, metabolic prenylation is slightly better studied in prokaryotic species. Specifically, in the later stages of peptidoglycan synthesis, transglycosylation results in the release of a prenyl substrate, undecaprenyl pyrophosphate (Apfel et al., 1999). This substrate is subsequently dephosphorylated to form bactoprenol which recycles cell wall polymers (Higashi et al., 1967). Additionally, quinone components of the electron transport chain (ETC), which transfer energy, require prenylation for function. Heme O is also a cofactor in the ETC, which mediates dioxygen reduction of heme-copper terminal oxidases. Heme O formation is catalyzed by a bacterial protoheme IX farnesyltransferase and the addition of a prenyl group to protoheme IX (Heme B) (Saiki et al., 1993).
In S. aureus, the crystal structure of a geranyltranstransferase (IspA) has previously been solved (Hosfield et al., 2004). This enzyme has been shown in E. coli to synthesize farnesyl pyrophosphates (FPPs), which is the central initiating step for prenylation within cells (Fujisaki et al., 1989; 1990). Given that the machinery for prenylation exists in S. aureus, we sought to explore the influence of this modification in this organism. We determined that the disruption of ispA in S. aureus does indeed lead to decreased FPP production and, quite surprisingly, pleiotropic reordering of cellular behavior, including impaired growth, major alterations in gene regulation, modified cell envelope architecture and altered responses to environmental stimuli. Therefore, we suggest that prenylation is potentially an underexplored and remarkably important process in bacteria, which governs and controls a wealth of key cellular functions.
Results
The geranyltranstransferase enzyme (IspA) is conserved in diverse bacterial species
A crystal structure for the S. aureus geranyltranstransferase (IspA) has previously been solved (Hosfield et al., 2004). To determine if IspA proteins (and thus the potential for prenylation) are well conserved in prokaryotic organisms, we performed BLAST analyses using the S. aureus IspA amino acid sequence (SAUSA300_1470). We determined that this enzyme is broadly conserved among a diverse array of bacterial species (Supporting Information Fig. S1). The conservation of the enzyme, not only among the Firmicutes but in Gram-negative species as well, suggests that prenylation is an important modification in bacterial cells.
IspA is responsible for FPP production in S. aureus cells
Geranyltranstransferases synthesize the condensation reaction required to generate FPP subunits from geranyl pyrophosphate and isopentenyl pyrophosphate (Fujisaki et al., 1986). As such, mutants lacking geranyltransferases should have impaired production of FPP. In order to confirm that the S. aureus geranyltranstransferase results in FPP synthesis, we analyzed levels of FPP in the wild-type (WT), ispA mutant and complemented strain. We determined that FPP levels in the WT strain and complemented strains are significantly higher than that of the ispA mutant (Fig. 1). This indicates, as predicted, that in the absence of ispA, S. aureus cells are unable to synthesize homeostatic levels of FPP.
Fig. 1.

IspA is required to synthesize farnesyl pyrophosphate in S. aureus cells. The amount of farnesyl pyrophosphate (FPP) in exponentially growing S. aureus cells was measured in the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains. Error bars are shown ± SEM; *p < 0.05 using a Student’s t-test.
Prenylation mutants of S. aureus display a small colony variant-like phenotype
We next set out to determine the effect of prenylation in S. aureus cells using an ispA-deficient strain. For complementation, we created a knock-in of ispA at its native chromosomal locus in the ispA mutant strain (see Materials and Methods). Upon initial phenotypic analysis of the prenylation mutant, we observed a striking alteration in cell size and color. Specifically, the absence of prenylation leads to a complete lack of pigmentation in S. aureus cells (Supporting Information Fig. S2). This lack of pigment is perhaps not surprising as it has previously been shown that the Staphyloxanthin biosynthetic pathway begins with the condensation of two FPPs to form dehydrosqualene by CrtM (Pelz et al., 2005). Growth of the mutant on TSA also revealed that ispA mutant colony size is smaller than the parental strain, presenting an small colony variant (SCV)-like phenotype. In order to determine if the smaller size results in a growth defect, growth curve analysis was performed on WT, ispA mutant and complemented strains under standard laboratory conditions in TSB. We determined that ispA mutants have a strong growth defect in TSB (Fig. 2A), with a significantly less robust exponential phase. Final densities are comparable with the parent and complemented strain in stationary phase; however, it takes at least 5 h longer to reach this phase compared with the parent. It should be noted that the decreased OD600nm values observed results from reduced CFU ml−1, rather than any effects of cell size on light scattering (Supporting Information Fig. S2C). Additionally, the growth defect displayed by the ispA mutant cannot be rescued by the increased aeration of cultures (1:10 flask volume−1 ratio vs 1:2.5 flask volume−1 ratio; Supporting Information Fig. S2D). Others have shown that the growth defect of SCVs can be chemically complemented by the addition of hemin, menadione or thymidine into growth media (von Eiff et al., 1997; Kahl et al., 2005; Norstrom et al., 2007). Importantly, such supplementation did not eliminate the SCV-like phenotype of the ispA mutant (Supporting Information Fig. S3), suggesting that this is a novel form of growth impairment, heretofore not reported. We next set out to explore if the growth defect observed for the prenylation mutant was more pronounced during growth in less replete media by profiling growth in amino acid-limiting and phosphate-limiting chemically defined media (CDM). A growth defect was detected in both amino-acid and phosphate-limiting media (Fig. 2B and C), in which ispA mutants exhibit delayed exponential growth, and failed to reach WT culture densities. To determine if these defects were complementable chemically, we supplemented media with individual amino acids (L-arginine, L-histidine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tryptophan, L-tyrosine and L-valine) that were limited in the original media. Although some benefit was seen during these experiments, ispA mutant growth improved only modestly (Supporting Information Fig. S4). Furthermore, the addition of L-cysteine and L-leucine slightly impaired the growth of the ispA mutant, again indicating that the absence of these two amino acids does not account for the growth defect. In the context of impaired growth in phosphate-limiting media, we hypothesized that this may result from a defect in ATP levels as S. aureus SCVs have previously been shown to contain decreased levels of this molecule (Balwit et al., 1994). An analysis of ATP levels proved this assertion to be correct, with the WT containing an average of 36.6 pmole μl−1 ATP, while the ispA mutant contained only 18.1 pmole μl−1 ATP (Fig. 3). Importantly, this defect is abrogated upon complementation. Collectively, it would appear that the disruption of prenylation results in a novel SCV-like phenotype.
Fig. 2.

The abrogation of prenylation leads to severely impaired growth. Optical densities (OD600nm) were measured for the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains during growth in TSB (A), amino acid-limiting CDM (B) or phosphate-limiting CDM (C). Error bars are shown ± SEM.
Fig. 3.
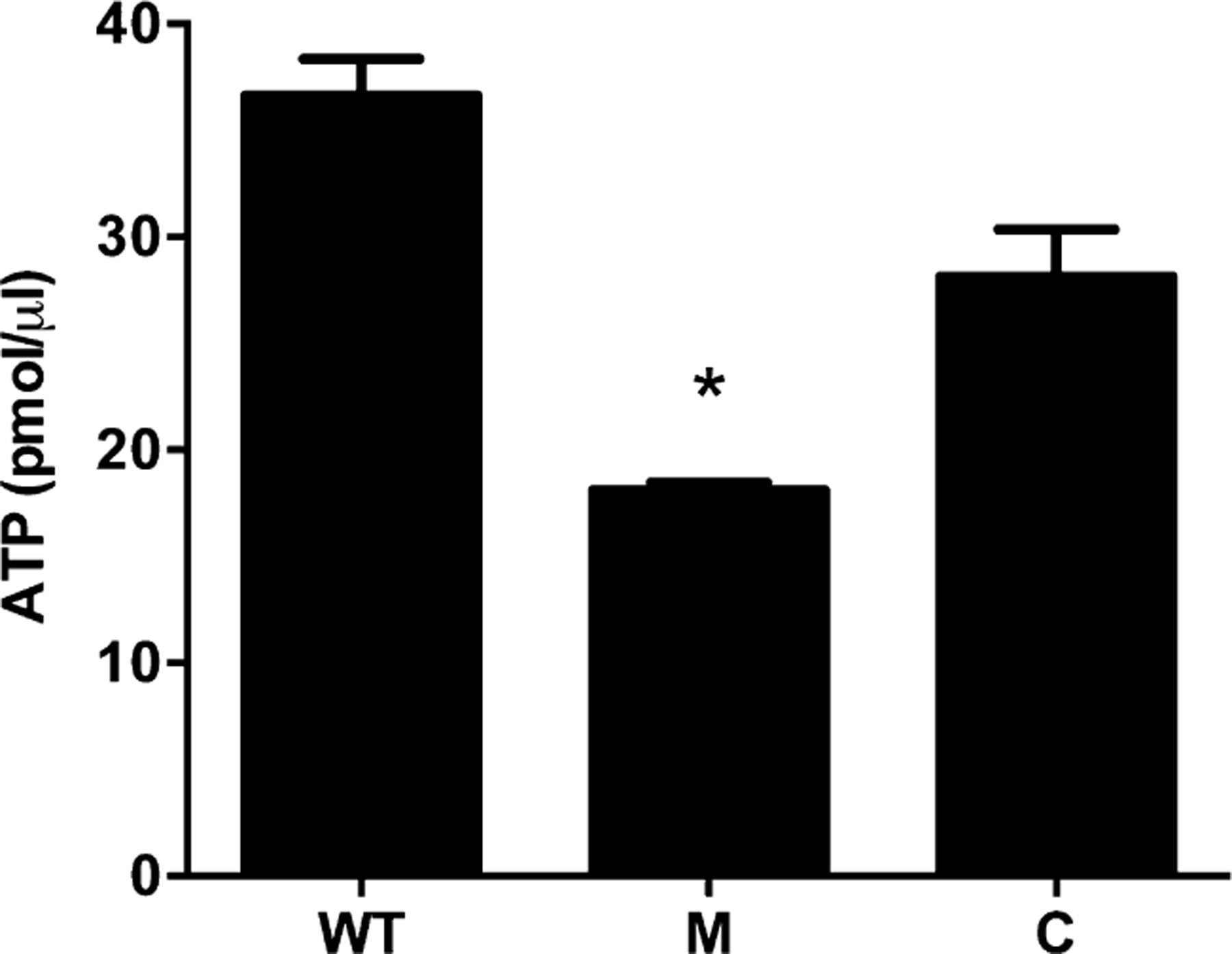
Prenylation defects result in lower cellular ATP levels. The amount of ATP in exponentially growing cells was measured for the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains. Error bars are shown ± SEM; *p < 0.05 using a Student’s t-test.
Exploring the influence of prenylation on S. aureus cells by transcriptomic analysis
Given that disruption of prenylation appears to have pleiotropic effects on S. aureus cells, we next set out to determine the extent of these influences using RNA-seq analysis. When comparing the ispA mutant to WT during exponential growth, we observed > 700 genes that were altered transcriptionally in the mutant at 2-fold or greater levels (Supporting Information Tables S1 and S2). Given that the number of alterations was so large, we sought to verify these findings. As such, genes from the data set were chosen at random, reflecting both increased and decreased transcription, and were analyzed using quantitative PCR (qPCR) (Supporting Information Fig. S5). Importantly, we noted similar fold changes in expression for all genes tested between the two datasets. Of the > 700 genes that were altered in expression, 290 were found to be down-regulated, while a further 412 were up-regulated in the mutant strain. These changes appear to be evenly distributed around the S. aureus genome (Fig. 4A) and affect every major ontology of protein function in the cell (Table 1, Fig. 4B). The most pronounced changes in expression were observed for genes involved in protein synthesis/degradation, with 85 such elements having altered transcription, followed by energy metabolism (82 genes). In addition, there were alterations in expression for numerous genes involved in cell envelope architecture, including cell surface proteins (38), membrane proteins (38), fatty acid (FA) metabolism (15) and lipoproteins (11). Finally, in addition to genes involved in metabolism and cell structure, there was altered expression of 54 regulatory elements and 45 virulence factors. This striking alteration in transcription for genes involved in basic cellular processes, regulation and virulence further supports the suggestion that prenylation is a central, and wide-reaching process, of significant importance to bacterial cells.
Fig. 4.

RNA-seq analysis reveals a major reprogramming of gene expression upon disruption of prenylation.
A. The outermost circle (yellow) is a heat map comparing changes in gene expression for the ispA mutant compared with wild-type (WT) strain. The middle circle (black) depicts reads per kilobase per million reads (RPKM) values for the wild type, and the inner circle (red) represents RPKM values for the ispA mutant.
B. The > 700 genes altered ≥ 2-fold in expression were organized in 20 ontologies (or unknown function).
Table 1.
Ontologies of genes that display alterations in expression in the ispA mutant.
| Ontology | Increased expression | Decreased expression | Total |
|---|---|---|---|
| Acetyltransferases | 4 | 5 | 9 |
| Cofactor biosynthesis | 14 | 5 | 19 |
| Cell surface proteins | 28 | 10 | 38 |
| DNA metabolism/repair | 7 | 10 | 17 |
| Fatty acid/phospholipid metabolism | 7 | 8 | 15 |
| Lipoproteins | 7 | 4 | 11 |
| Membrane proteins | 19 | 19 | 38 |
| Metabolism | 47 | 35 | 82 |
| Mobile elements | 5 | 3 | 8 |
| Nitrate/nitrite proteins | 0 | 5 | 5 |
| Phage proteins | 22 | 1 | 23 |
| Proteases | 9 | 15 | 24 |
| Protein synthesis/degradation | 53 | 32 | 85 |
| Nucleic acid biosynthesis | 16 | 5 | 21 |
| Regulators | 28 | 26 | 54 |
| Replication and division | 8 | 3 | 11 |
| Stress response | 20 | 9 | 29 |
| Transcription | 11 | 3 | 14 |
| Toxins/virulence factors | 23 | 22 | 45 |
| Transport/binding proteins | 49 | 21 | 70 |
| Unknown function | 35 | 49 | 84 |
| Total | 412 | 290 | 702 |
A functional prenylation system is required to protect cells from killing by oxidative stress
Nonpigmented S. aureus cells lack Staphyloxanthin, which is an antioxidant that provides protection against oxidative stress. In addition, our RNA-seq data showed diminished expression of genes encoding oxidative response proteins, such as thioredoxin, two thioredoxin family proteins, methionine sulfoxide reductase MsrA2 and superoxide dismutase. We next sought to determine if ispA mutants were susceptible to killing by a variety of oxidative stress agents, including H2O2, and the superoxide-generating compounds pyrogallol, cadmium chloride and hemin (Hassoun and Stohs, 1996) (Fig. 5). After 40 min exposure to cadmium chloride, we observed 79.8% recovery of the WT, whilst the ispA mutant returned only 18.5%. Similarly, following a 5 minute exposure to hydrogen peroxide, the percent recovery of the wild type was 33.9%, yet only 0.83% for the mutant. Using pyrogallol (1 h exposure), we observed a 76.9% recovery of the WT strains, but only 0.98% for the mutant. Lastly, growth analysis using hemin reveals that ispA mutants have an even more pronounced growth defect than under standard conditions, with a highly aberrant exponential phase. Importantly, complementation with ispA restored the survival defect to parental levels in each condition tested. Taken together, these results demonstrate that ispA mutants display marked sensitivity to oxidative stress, likely resulting from impaired pigment production and reduced expression of key stress resistance proteins.
Fig. 5.

ispA mutants have elevated sensitivity to oxidative stress.
Viability of the wild-type (WT), ispA mutant(M) and ispA complemented (C) strains was assessed in the presence of 20 mM cadmium chloride (A), 1.4 M hydrogen peroxide (B), 40 mM pyrogallol (C) and 20 μM hemin (D). CFU counts were determined for strains pre-and post-exposure to oxidative stress agents A–C, while optical density (OD600nm) was used to determine growth in hemin (D). All data are the average of three independent experiments; error bars are shown ± SEM; *p < 0.05 using a Student’s t-test.
ispA mutants are more resistant to alkaline shock
An interesting observation from our RNA-seq data was the increased expression of all three alkaline shock proteins in the S. aureus genome (asp23, SAUSA300_1474 and SAUSA300_1118) in the ispA mutant. In order to determine if the increased expression of these genes provides a survival benefit, an alkaline shock assay was performed. In response to shock of pH 11.8, percent recovery of ispA mutants was 18.9-fold greater that the WT and ispA complemented strains (Fig. 6). These findings tend to suggest that the increased production of Asp23-like proteins does lead to enhanced survival upon pH increase.
Fig. 6.

Disrupting prenylation leads to an increased resistance toward alkaline shock. Viability of the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains was assessed in response to alkaline shock. CFU counts were determined for strains pre- and post-exposure to pH 11.8 for 15 minutes and are averaged from three independent experiments. Error bars are shown ± SEM; *p < 0.05 using a Student’s t-test.
Absence of prenylation leads to increased resistance to aminoglycosides and antimicrobial peptides
Since S. aureus SCVs are known to have increased resistance to aminoglycosides antibiotics (Balwit et al., 1994), we explored sensitivity of the ispA mutant to such agents. Upon analysis, we determined that while the parental strain had a minimum inhibitory concentration (MIC) for gentamicin of 5 μg ml−1, the mutant was more than 2-fold resistant to this agent (MIC = 12.5 μg ml−1). Similarly, the neomycin MIC for the ispA mutant is 6.25 mg ml−1, which is 2.5-fold higher than that of the WT (2.5 mg ml−1). Finally, the kanamycin MIC for the ispA mutant is 12.5 mg ml−1, which is 2-fold higher than that of the WT (6.25 mg ml−1). In connection with such phenotypes, SCVs have also been shown to display enhanced resistance to cationic antimicrobial peptides, caused by an inability of positively charged molecules to penetrate their membranes (Balwit et al., 1994). Upon analysis using polymyxin B and LL-37, we found this observation to be the case, with ispA mutants proving more resistant than WT strains over increasing drug concentrations (Fig. 7). For polymyxin B, the greatest difference occurs in the presence of 100 μg ml−1 of the drug, wherein survival of the ispA mutant (418.3%) was 6.6-fold higher than the WT strain (65.7%). Similarly, maximal differences occurred in the presence of 15 μg ml−1 LL-37 where the survival of the ispA mutant (102.9%) was 3.6-fold higher than the WT strain (28.6%). Taken together, these results indicate ispA mutants exhibit similar resistances to positively charged molecules as previously described for other S. aureus SCVs.
Fig. 7.

The absence of prenylation results in resistance to antimicrobial peptides.
Percent recovery of the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains were assessed in the presence of increasing concentrations of polymyxin B (A) and LL-37 (B). Error bars are shown ± SEM; *p < 0.05 using a Student’s t-test.
Prenylation mutants of S. aureus have increased sensitivity to cell wall-targeting antibiotics and marked alterations in envelope composition
Although S. aureus SCVs have also been shown to have elevated resistance to cell wall-targeting antibiotics (Looney, 2000), we found the reverse to be true for the ispA mutant, where we actually observed increased sensitivity to β-lactam antibiotics. Specifically, the ampicillin MIC for the WT was 102 μg ml−1, which was 4.1-fold higher than that of the ispA mutant (25 μg ml−1). Additionally, the WT MIC for cefotaxime was 10 μg ml−1 compared with 5 μg ml−1 for the mutant. Most notably, the penicillin-G MIC for the WT was 617 μg ml−1, which is 12.3-fold higher than that of the ispA mutant (50 μg ml−1). These sensitivities may be due in part to the down-regulation of genes with known roles in resistance to antibiotics, including SAUSA300_2087 (a HMRA peptidase) and SAUSA300_1797 (an XRE family regulator) (Botelho et al., 2011; Gebhard, 2012). To determine if the sensitivity to cell wall-targeting agents was mediated by notable changes in cell ultrastructure, we next analyzed ispA mutants for physically altered cellular morphology using transmission electron microscopy (TEM). Imaging of the mutant in comparison with the parent and complement strains revealed that the ablation of prenylation leads to a diffuse and less defined cell envelope, particularly at the septum of dividing cells (Fig. 8). These differences in cell envelope architecture may be explained by the vast number of cell envelope genes (> 80) which exhibit alteration in expression. Thus, the sensitivity of the ispA mutant to cell wall-targeting antibiotics appears to be mediated by alterations in cell envelope composition.
Fig. 8.

Prenylation is necessary to maintain normal cell envelope biosynthesis.
Transmission electron micrographs are shown for exponentially growing wild-type (WT) (A), ispA mutant (B) and ispA complemented (C) strains. Images are representative of at least 10 different frames, and are typical for each cell population.
The disruption of prenylation leads to alterations in membrane fatty acid composition
To explore these alterations in the cell envelope further, we next performed membrane FA profiling. This was done in part because of the marked differences in cell wall ultrastructure observed in ispA mutants and also because our RNA-seq data revealed alterations in the expression of 15 genes involved in FA synthesis and metabolism. Therefore, we determined membrane FA composition of the ispA mutant, WT and ispA complemented strains using FA methyl ester (FAME) analysis. Strikingly, we observed increased production of longer chained FAs in the ispA mutant and a shift away from shorter chain FA synthesis (Fig. 9). Specifically, the ispA mutant lacks any methyl 15-methylhexadeconate, which is present in the WT strain, yet possesses methyl nona-decanoate, which is completely absent from the parent. Additionally, methyl eicosanoate is 3.4-fold more abundant in the ispA mutant compared with the WT strain. Importantly, all such changes were reversed upon complementation analysis. We propose that this shift toward longer FA chains in the ispA mutant may be a possible mechanism in which membrane architecture is reorganized to combat apparent cell wall weaknesses in the absence of prenylation.
Fig. 9.

Fatty acid composition is altered in the membranes of prenylation mutants. FAME composition of the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains was assessed and measured as percent of total fatty acid composition. Data presented are from at least three independent replicates.
The altered membrane composition of ispA mutants leads to increased membrane fluidity
FAME analysis demonstrates alterations in the composition of ispA mutant membranes that would likely lead to increased membrane fluidity. Indeed, Gram-negative bacteria have been shown to alter FA stereochemistry and chain length in order to stabilize membrane fluidity resulting from weakening of the cell wall (Massa et al., 1988). Accordingly, cell survivability was assessed in the presence of free unsaturated oleic acid, which more easily insert into membranes with increased fluidity, leading to increased cell death. As expected, when exposed to oleic acid, the ispA mutant displayed decreased recovery compared with the WT (Fig. 10A). After 1 h incubation, we observed 18.2-fold greater resistance of the WT compared with the mutant strain that was abrogated upon complementation. To validate these findings, we next tested susceptibility to organic solvents as cells with increased membrane fluidity have been shown to be less susceptible to killing by such agents (Nielsen et al., 2005). Again, as predicted, upon exposure to toluene, ispA mutants exhibit an increase in survival compared with the parent strain (Fig. 10B). After 1 h incubation in toluene, the ispA mutant displayed 8.5-fold greater resistance that was again reversed upon complementation. Interestingly, a study performed on rodZ mutants of E. coli determined that mutations in ispA restored the ability of rodZ mutants to grow at low temperatures as a consequence of alterations in cell membrane fluidity and/or wall composition (Shiomi and Niki, 2011). In addition, our RNA-seq analysis revealed strong upregulation of two cold shock proteins (CspA and CspC) in the mutant strain. As such, we performed survival studies at 4°C to assess whether these collective affects influence the ability of the ispA mutant to resistant lower temperatures. Interestingly, whilst we did not observe enhanced survival of the ispA mutant compared with the parent under these conditions, we did note that the typical growth defect observed at 37°C was completely absent (Supporting Information Fig. S6). Therefore, it would appear that reduced temperatures can rescue the prenylation-mediated growth defects of S. aureus cells, likely resulting, at least in part, from alterations in FA composition and the upregulation of stress response genes.
Fig. 10.

Membrane fluidity is altered in the absence of prenylation. Viability of the wild-type (WT), ispA mutant (M) and ispA complemented (C) strains was assessed in the presence of oleic acid (A) and toluene (B). CFU counts were determined at 15-minute intervals and are averaged from three independent experiments. Error bars are shown ± SEM; *p < 0.05 using a Student’s t-test.
Bioinformatic exploration for putative prenylated proteins
To explore whether protein prenylation putatively occurs in prokaryotes, we performed a bioinformatic analysis of the S. aureus proteome to search for translated products containing the characteristic eukaryotic ‘CAAX’ motif at their C-terminus. Our analysis revealed only two such proteins: TrmE, a tRNA modification GTPase, and SAUSA300_1902, a conserved hypothetical protein that is homologous to 3-carboxy-cis, cis-muconate lactonizing enzymes. To assess whether this paucity of CAAX-motif proteins is common to other bacteria, we performed the same studies with the B. subtilis and E. coli proteomes. We again found a very small number of proteins with this characteristic motif at their C-terminus (Supporting Information Table S3). This dearth of proteins containing the prototypical CAAX-motif in prokaryotes suggests that either proteins are not prenylated in bacterial organisms or that such modifications occur at entirely different protein recognition sequences.
Discussion
Herein we present the exploration of prenylation, and its role in bacterial cells, using S. aureus as a model. We observed a wealth of alterations in cellular behavior, detailed as follows (and in Fig. 11). Of key importance is the finding that mutant strains lacking IspA are unable to synthesize FPP to WT levels. It should be noted that the disruption of ispA does not completely abrogate FPP production in S. aureus, which mirrors findings from E. coli (Fujisaki et al., 2005). These reduced, but not entirely absent, FPP levels are perhaps not unexpected as the complete loss of FPP in bacterial cells would be lethal since this compound is required for the synthesis of vital cellular components, including quinones and heme O of the ETC, and subunits of the cell wall (Higashi et al., 1967; Bentley and Meganathan, 1982; Saiki et al., 1993; Nowicka and Kruk, 2010). It is believed that in the absence of IspA, small amounts of FPP are generated by moonlighting functions of other prenylation enzymes (e.g. octaprenyl synthase or undecaprenyl synthase). Such levels, however, are far lower than those produced by IspA, thus leading to the pleiotropic effects observed upon ispA disruption.
Fig. 11.

Model for the role of prenylation within S. aureus cells
The most apparent outcome from our study of a prenylation deficient ispA mutant is its complete lack of pigmentation. Producing the precursor building blocks for Staphyloxanthin is dependent of a functional geranyltranstransferase, explaining the absence of pigmentation upon disruption of this enzyme (Pelz et al., 2005). Staphyloxanthin is also an antioxidant, which has been shown to contribute to survival in response to oxidative stresses, including in vitro killing by reactive oxygen species (hydrogen peroxide, superoxide radical, hydroxyl radical, hypochloride) and in vivo killing by oxidative bursts exhibited by neutrophils and whole blood (Liu et al., 2005; Clauditz et al., 2006). Logically, we also observe similar sensitivity in the ispA mutant, which is likely explained by this lack of pigment, and the decreased expression of several genes (thioredoxin, two thioredoxin family proteins, methionine sulfoxide reductase MsrA2 and superoxide dismutase) involved in oxidative stress responses in the mutant strain.
Interestingly, ispA mutants also display classical hallmarks of S. aureus SCVs, including smaller colony size, a lack of pigmentation and impaired growth (Looney, 2000; Melter and Radojevic, 2010; Atalla et al., 2011; Day, 2013; Garcia et al., 2013). Importantly, the prenylation-deficient SCV phenotype observed appears to be a unique one and does not result from deficiencies in menadione, hemin or thymidine synthesis. Additionally, characteristic SCV phenotypes such as ‘fried egg’ colony morphology on TSA or absence of hemolysis on blood agar are not present in ispA mutants (data not shown) (Kahl et al., 2003). In the context of the growth defect observed, this mirrors findings from prenylation mutants in eukaryotes. Specifically, Candida calbrata mutants of geranyltranstransferase ERG20 and prenyltransferase RAM2 exhibit impaired growth in both laboratory media and in murine kidneys (Nakayama et al., 2011).
Transcriptomic analyses performed on the ispA mutant revealed profound effects on gene expression in response to the ablation of prenylation, with > 700 genes altered in transcription. Several similar analyses have been performed on both laboratory and clinical SCVs, focusing particularly on the expression of virulence determinants. Quantitative PCR and Northern blot analyses performed on clinically derived thymidine-dependent SCVs and their isogenic parent strains revealed a decrease in expression of asp23, sarA, hld and hla, and increased expression of spa (Kahl et al., 2005). Conversely, similar studies performed by Moisan et al. (2006) on clinical SCVs demonstrate an increase in expression of asp23 and sarA, indicating the variation of expression among different clinical isolates. They also analyzed the expression of these genes in hemB mutants and determined that expression of asp23, sarA, hld and hla are all decreased. RNA-seq analysis of ispA mutants revealed an increase in expression of asp23 and sarA, similar to results of the clinically derived SCVs analyzed by Moisan et al., yet an increase in spa expression similar to thymidine-dependent SCVs. These alterations in genetic profiles of previously described SCVs and the ispA mutant further confirm that the SCV-like phenotype presented herein does not conform to those already documented.
We also investigated the effects of numerous antibiotics on survival of ispA mutants in order to determine if they exhibit altered resistances, similar to SCVs. Most notably, and akin to other SCVs, ispA mutants are resistant to aminoglycosidic antibiotics. This resistance to aminoglycosides by SCVs has been attributed to the inability of positively charged molecules to penetrate the cell membrane due to a reduced electrochemical gradient and lower ATP levels (Balwit et al., 1994). The resistance of the ispA mutant to aminoglycosides is perhaps not surprising as prenyl groups are required to synthesize components of the ETC, such as menaquinones and heme O (Bentley and Meganathan, 1982). The absence of these ultimately leads to less efficient ATP production, which we also observe in our ispA mutant strain. In contrast to these findings, we determined that, unlike typical SCVs, ispA mutants are actually more sensitive to cell wall-targeting antibiotics than the parental strain (Looney, 2000). This sensitivity to cell wall-targeting antibiotics may be the result of a weakened cell wall. Indeed, this is supported by the fact that prenyl groups are required for the formation of bactoprenol; thus, any defect in prenylation would impair the recycling and generation of cell wall precursors. Interestingly, TEM of ispA mutants did not reveal thickening of cell walls seen in previously described SCVs (Adler et al., 2005), which is believed to mediate SCV resistance to cell wall-targeting antibiotics. Instead, ispA mutants display a thinner and more diffuse cell envelope perhaps resulting from disruption of normal transglycosylation, which requires the prenyl substrate undecaprenyl pyrophosphate (Apfel et al., 1999). Taken together, these observations reveal that in the absence of adequate levels of farnesyl substrates, normal membrane and wall synthesis are unable to proceed.
When further investigating the effects of prenylation on cell envelope architecture, we observed increased fluidity of the ispA mutant strain. This is seemingly attributed to changes in FA composition, which we observed upon FAMEs analysis. Specifically, we noted that the ispA mutant shifts FA production and incorporation toward longer-chain FAs, suggesting a possible mechanism by which membrane architecture is reorganized to combat cell wall weaknesses. Many studies have been performed that elucidate the mechanisms in which bacteria alter membrane fluidity as a response to a variety of environmental stressors. For example, E. coli decreases its membrane fluidity by increasing the number of unsaturated FAs in its cell membrane in response to cold temperatures, while the reverse is true in response to increased growth temperatures (Marr and Ingraham, 1962; Sinensky, 1974; Casadei et al., 2002). In addition to alteration in FA profile, a lack of Staphyloxanthin has also been implicated in this process as polar carotenoids have been demonstrated to be important in maintaining membrane fluidity (Gruszecki and Strzal·ka, 1991; Subczynski et al., 1992; Wisniewska and Subczynski, 1998; Wisniewska et al., 2006). Overall, the alteration in membrane fluidity in ispA mutants likely is a response to alterations in cell wall architecture, which leads to the adjustment of FA composition.
Interestingly, many, if not all, of the changes in cellular behavior observed in the ispA mutant of S. aureus are explained by the ablated prenylation of metabolites. Further to this, our bioinformatic analysis revealed a significant underrepresentation of the characteristic CAAX-motif found in prenylated proteins compared with eukaryotic cells, wherein approximately 2–5% of translated products are prenylated (Gao et al., 2009; Wollack et al., 2009). This suggests that protein prenylation might not occur in prokaryotic cells, or that it proceeds via a mechanism that is distinct from their eukaryotic counterparts. Indeed, a lone example of prenylation exists in bacteria: The pheromone ComX in Bacillus species has a geranyl modification on a tryptophan residue, rather than the prototypical cysteine observed in eukaryotes (Okada et al., 2008; Tsuji et al., 2011). Intriguingly, when we studied the S. aureus genome for components of the protein prenylation machinery, we observed five M79 family CAAX-processing proteases (only three of which appear to be functional from studying the conservation of catalytic residues), which serve in eukaryotes to cleave the ‘-AAX’ motif from the C-terminus of prenylated proteins. In addition, we found a putative isoprenylcysteine carboxyl methyltransferase, which, in eukaryotes, transfers a methyl group to the newly exposed, C-terminal cysteine residue of farnesylated and geranylgeranylated proteins. Interestingly, upon searching the genome of other bacterial species, we observed strong conservation of these putative protein prenylation components in other organisms, particularly in Gram-positives (Supporting Information Fig. S7). Therefore, it is possible that prenylation exists as a protein PTM in bacterial cells; however, if it does, it likely occurs by a completely novel and as yet undescribed mechanism. Work to uncover if proteins are prenylated in prokaryotes, and the process by which this occurs, is currently underway in our laboratory.
Experimental procedures
Bacterial strains, plasmids and growth conditions
Optical density (OD600nm) of S. aureus strains was measured using a Synergy 2 plate reader (Bio-Tek) every hour for at least 8 h in tryptic soy broth (TSB; 1.7% tryptone, 0.3% soytone, 0.5% sodium chloride, 0.25% dextrose, 0.25% potassium phosphate dibasic), amino acid-limiting CDM or phosphate-limiting CDM. Additional validation was performed in flasks (100 ml TSB with a 12.5 flask volume−1 ratio), as described previously (Shaw et al., 2008). Amino acid and phosphate-limiting CDM were used as described previously (Clements and Foster, 1998). When required, antibiotics were added to media at the following concentrations: ampicillin, 100 μg ml−1 (E. coli); chloramphenicol, 5 μg ml−1 (S. aureus); erythromycin, 5 μg ml−1 (S. aureus); lincomycin, 25 μg ml−1 (S. aureus); and anhydrotetracycline, 1 μg ml−1 (S. aureus).
Construction of an ispA mutant strain
An ispA mutant was obtained from the Nebraska transposon mutant library (Fey et al., 2013). The USA300 JE2 mutant (NE1447) was used to generate a phage lysate to transduce strain USA300 HOU (Kolar et al., 2011) via Φ11-mediated transduction. The resulting strain CNK1726 was confirmed by PCR analysis using OL2321, located upstream of ispA, and transposon-specific primer OL1472 (the sequence of all primers can be found in Supporting Information Table S4).
Construction of an ispA complement strain
A knock-in ispA complement strain was constructed by first amplifying the ispA coding region including approximately 500 bp upstream and downstream of the gene using primer pair OL2664/OL2665. This amplification product was subsequently cloned into pJB38 (Bose et al., 2013), creating plasmid pCNK1802. E. coli DC10B (Monk et al., 2012) was transformed with CNK1802 and confirmed using genespecific primer OL2323 and plasmid-specific primer OL2381. USA300 HOU ispA::bursa aurealis was electroporated at 30°C with pCNK1802 and confirmed again by PCR. Subsequently, the confirmed transformant was incubated at 42°C to force integration of the plasmid into the chromosome as previously described (Bose et al., 2013). Finally, anhydrotetracycline was used in order to select for cells that underwent double recombination events, thus restoring ispA at its native chromosomal site. Clones were confirmed by PCR analysis using ispA-flanking primer pair OL 2321/2322, generating strain CNK1803.
FPP analysis
FPP was extracted in triplicate from WT, ispA mutant and complemented strains that were standardized by OD600nm as previously described (Vallon et al., 2008). An FPP standard (Sigma) was dissolved in acetonitrile pyrophosphate at a concentration of 2.5, 1.0, 0.5, 0.25, 0.10, 0.05, 0.025 and 0.01 μl ml−1 to prepare a standard curve. Samples from the WT, ispA mutant and complemented strain were then dried under argon and extracted with 1 ml hexane. Particulate matter was removed by filtering through Celite and glass wool, and the hexane was removed under argon. Samples were redissolved in 500 μl of acetonitrile, and the samples were further filtered with 0.2 μm filters prior to analysis. FPP was measured by LC-MS/MS using an HPLC coupled to a TSQ Quantum Ultra (Thermo Scientific) with an electrospray ionization source. A Higgins Analytical Inc. C18 column (250 × 1.0 mm i.d., with a 5 μm particle size) was utilized for the separation of analytes, with the column heated to 30°C. The mobile phase, consisting of 20 mmol L−1 ammonium bicarbonate in a 0.1% triethyl amine aqueous solution (Solution A) and a water/acetonitrile (1/9 v/v) including a 0.1% triethylamine (Solution B), was pumped at a rate of 50 μl min−1. The linear gradient was optimized at 1–100% solution B over 10 min, when 100% B was pumped isocratically for an additional 2 min. Aliquots were then injected onto the column to generate the standard curve and for analysis of bacterial extracts. FPP was analyzed in negative mode using single reaction monitoring, focusing on the parent ion’s transition from m/z 381.1 to a daughter ion of m/z 79.1 utilizing a collision energy of 35 V with a scan time of 1 s. The molecules were ionized using a discharge voltage of 4000 V, capillary temperature of 300 °C and a sheath gas pressure of 20 psi.
ATP measurement assay
Synchronized USA300 WT, ispA mutant and complemented strains were grown for 3 h before their OD600nm was standardized, and the cells pelleted by centrifugation. Cells were then lysed in ATP buffer (BioVision) by mechanical disruption using 0.1 mm glass beads and a Mini BeadBeater-16 (BioSpec). ATP quantification was performed using an ATP colorimetric/fluorometric assay kit (BioVision) according to the manufacturer’s protocol. Data are presented from three independent experiments.
RNA-seq analysis
RNA-seq was performed on exponentially growing USA300 WT and ispA mutant strains as described by us previously (Weiss et al., 2014).
qPCR
qPCR was performed as previously described (Kolar et al., 2011). Primer pairs (Supporting Information Table S4) were used to analyze expression of hrtA, rplM, sepF, ureA, crtM, spa, SAUSA300_1966 and SAUSA300_2129. Control primers for 16S rRNA were used as previously described (Koprivnjak et al., 2006).
MICs
The MICs of antibiotics were determined as previously described (Burda et al., 2012). The MIC was defined as the lowest concentration of antibiotic that inhibited bacterial growth as determined by absence of turbidity in microtiter wells from at least three independent experiments.
Stress survival assays
Exponentially growing USA300 WT, ispA mutant and complemented strains were washed three times with PBS. Cells were resuspended in PBS, and oxidative stressors (cadmium chloride, hydrogen peroxide, pyrogallol or hemin) were added to final concentrations of 20 mM, 1.4 M, 40 mM and 20 μM respectively. Cultures were incubated shaking at 37°C, and the percent recovery was determined by comparing pre-exposure CFU ml−1 counts to postexposure CFU ml−1 counts for cadmium chloride, hydrogen peroxide and pyrogallol assays. OD600nm of cultures was measured every hour for 13 h for the hemin assay. Data are presented from at least three independent experiments.
Alkaline shock assay
Exponentially growing USA300 WT, ispA mutant and complemented strains were washed three times with PBS. Cells were resuspended in PBS adjusted to pH 11.8 with 1M NaOH. Cultures were incubated shaking at 37°C, and the percent recovery was determined by comparing pre-exposure CFU ml−1 counts to 15 min postexposure CFU ml−1 counts. Data are presented from at least three independent experiments.
Oleic acid and toluene survival assays
Exponentially growing USA300 WT, ispA mutant and complemented strains were washed three times with PBS. Cells were resuspended in 0.01% (v/v) oleic acid in PBS or 1.25% (v/v) toluene in TSB. Cultures were incubated shaking at 37°C, and the percent recovery was determined by comparing pre-exposure CFU ml−1 counts to CFU ml−1 at 15-minute intervals postincubation with either agent. Data are presented from three independent experiments.
FAME analysis
Membrane lipids were isolated from USA300 WT, ispA mutant and complemented strains as previously described (Grundling and Schneewind, 2007). The aqueous supernatant containing the lipids was partitioned with hexane, the lipophilic layer removed and dried under argon. The bacterial FAs were methylated with a freshly prepared ethereal diazomethane solution (500 μl, 1.66 mM). The reaction was allowed to proceed, at ambient temperature, until the solution turned colorless (ca. 30 min). The excess ether was removed under argon, and the bacterial FAMEs were redissolved in hexane (500 μl). The relative FAME composition was determined via gas chromatography–mass spectrometry (GC-MS) analysis and compared with commercially available FAMEs in methyl caproate (Supelco, 47080-U). Standards and extracts were analyzed on an Agilent 7980AGC interfaced to an Agilent 7000 series QqQ mass spectrometer operating in electron ionization. Injections of 2 μl of the bacterial extract solution were vaporized on the preheated splitless inlet at 300°C, then introduced onto an HP-5ms column (30 m × 0.25 mm i.d.) using a 17.5 min temperature gradient (initial oven temperature of 150°C, held for 4 min, heated to a final temperature of 230°C at a rate of 4°C min−1, then held at final temperature for a further 3 min). Helium was used as a carrier gas at a constant flow rate of 1 ml min−1. The FAMEs present in each sample were determined by comparison of retention times and mass spectroscopy fragmentation patterns obtained from a standard mix of bacterial acid methyl esters.
TEM
USA300 WT, ispA mutant and complemented cultures were grown to exponential phase (3 h), prepared for electron microscopy analysis and photographed at the University of South Florida Department of Integrative Biology Microscopy Core Facility as previously described (Kolar et al., 2011).
Supplementary Material
Acknowledgements
We would like to thank Edward Haller, from the Microscopy Core Facility in the Department of Integrative Biology at the University of South Florida, for his help with this study. Strain NE1447 was obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program, supported under NIAID, NIH Contract No. HHSN272200700055C. This study was supported in part by grant AI080626 (L. N. S.) from the National Institute of Allergies and Infectious Diseases.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Adler H, Schraner EM, Frei R, and Wild P (2005) Ultrastructure of a clinical isolate of Staphylococcus aureus small colony variant and its revertant. Microsc Microanal 11: 982–983. [Google Scholar]
- Apfel CM, Takacs B, Fountoulakis M, Stieger M, and Keck W (1999) Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential uppS gene. J Bacteriol 181: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalla H, Gyles C, and Mallard B (2011) Staphylococcus aureus small colony variants (SCVs) and their role in disease. Anim Health Res Rev 12: 33–45. [DOI] [PubMed] [Google Scholar]
- Balwit JM, van Langevelde P, Vann JM, and Proctor RA (1994) Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis 170: 1033–1037. [DOI] [PubMed] [Google Scholar]
- Bentley R, and Meganathan R (1982) Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev 46: 241–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JL, Fey PD, and Bayles KW (2013) Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol 79: 2218–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho TO, Guevara T, Marrero A, Arede P, Fluxa VS, Reymond JL, et al. (2011) Structural and functional analyses reveal that Staphylococcus aureus antibiotic resistance factor HmrA is a zinc-dependent endopeptidase. J Biol Chem 286: 25697–25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda WN, Fields KB, Gill JB, Burt R, Shepherd M, Zhang XP, and Shaw LN (2012) Neutral metallated and meso-substituted porphyrins as antimicrobial agents against gram-positive pathogens. Eur J Clin Microbiol Infect Dis 31: 327–335. [DOI] [PubMed] [Google Scholar]
- Cain JA, Solis N, and Cordwell SJ (2014) Beyond gene expression: the impact of protein post-translational modifications in bacteria. J Proteomics 97: 265–286. [DOI] [PubMed] [Google Scholar]
- Casadei MA, Manas P, Niven G, Needs E, and Mackey BM (2002) Role of membrane fluidity in pressure resistance of Escherichia coli NCTC 8164. Appl Environ Microbiol 68: 5965–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauditz A, Resch A, Wieland KP, Peschel A, and Gotz F (2006) Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74: 4950–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements MO, and Foster SJ (1998) Starvation recovery of Staphylococcus aureus 8325–4. Microbiology 144 (Part 7): 1755–1763. [DOI] [PubMed] [Google Scholar]
- Day M (2013) Yeast petites and small colony variants: for everything there is a season. Adv Appl Microbiol 85: 1–41. [DOI] [PubMed] [Google Scholar]
- Dumelin CE, Chen Y, Leconte AM, Chen YG, and Liu DR (2012) Discovery and biological characterization of geranylated RNA in bacteria. Nat Chem Biol 8: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, and Gotz F (1997) A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol 179: 4706–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade PJ, and Hughes AL (2007) Regulation of sterol synthesis in eukaryotes. Annu Rev Genet 41: 401–427. [DOI] [PubMed] [Google Scholar]
- Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, and Bayles KW (2013) A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4: e537–e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki S, Nishino T, and Katsuki H (1986) Isoprenoid synthesis in Escherichia coli. Separation and partial purification of four enzymes involved in the synthesis. J Biochem 99: 1327–1337. [DOI] [PubMed] [Google Scholar]
- Fujisaki S, Nishino T, Katsuki H, Hara H, Nishimura Y, and Hirota Y (1989) Isolation and characterization of an Escherichia coli mutant having temperature-sensitive farnesyl diphosphate synthase. J Bacteriol 171: 5654–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki S, Hara H, Nishimura Y, Horiuchi K, and Nishino T (1990) Cloning and nucleotide sequence of the ispA gene responsible for farnesyl diphosphate synthase activity in Escherichia coli. J Biochem 108: 995–1000. [DOI] [PubMed] [Google Scholar]
- Fujisaki S, Takahashi I, Hara H, Horiuchi K, Nishino T, and Nishimura Y (2005) Disruption of the structural gene for farnesyl diphosphate synthase in Escherichia coli. J Biochem 137: 395–400. [DOI] [PubMed] [Google Scholar]
- Gao J, Liao J, and Yang GY (2009) CAAX-box protein, prenylation process and carcinogenesis. Am J Transl Res 1: 312–325. [PMC free article] [PubMed] [Google Scholar]
- Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, et al. (2013) Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J Antimicrob Chemother 68: 1455–1464. [DOI] [PubMed] [Google Scholar]
- Gebhard S (2012) ABC transporters of antimicrobial peptides in Firmicutes bacteria - phylogeny, function and regulation. Mol Microbiol 86: 1295–1317. [DOI] [PubMed] [Google Scholar]
- Grundling A, and Schneewind O (2007) Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J Bacteriol 189: 2521–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszecki WI, and Strzal·ka K (1991) Does the xanthophyll cycle take part in the regulation of fluidity of the thylakoid membrane? Biochim Biophys Acta 1060: 310–314. [Google Scholar]
- Hassoun EA, and Stohs SJ (1996) Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology 112: 219–226. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Strominger JL, and Sweeley CC (1967) Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci USA 57: 1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosfield DJ, Zhang Y, Dougan DR, Broun A, Tari LW, Swanson RV, and Finn J (2004) Structural Basis for Bisphosphonate-mediated Inhibition of Isoprenoid Biosynthesis. J Biol Chem 279: 8526–8529. [DOI] [PubMed] [Google Scholar]
- Kahl BC, Belling G, Reichelt R, Herrmann M, Proctor RA, and Peters G (2003) Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J Clin Microbiol 41: 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl BC, Belling G, Becker P, Chatterjee I, Wardecki K, Hilgert K, et al. (2005) Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect Immun 73: 4119–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar SL, Nagarajan V, Oszmiana A, Rivera FE, Miller HK, Davenport JE, et al. (2011) NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 157: 2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivnjak T, Mlakar V, Swanson L, Fournier B, Peschel A, and Weiss JP (2006) Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J Bacteriol 188: 3622–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, et al. (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney WJ (2000) Small-colony variants of Staphylococcus aureus. Br J Biomed Sci 57: 317–322. [PubMed] [Google Scholar]
- Marr AG, and Ingraham JL (1962) Effect of temperature on the composition of fatty acids in Escherichia Coli. J Bacteriol 84: 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa EM, Lopez Vinals A, and Farias RN (1988) Influence of unsaturated fatty acid membrane component on sensitivity of an Escherichia coli fatty acid auxotroph to conditions of nutrient depletion. Appl Environ Microbiol 54: 2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melter O, and Radojevic B (2010) Small colony variants of Staphylococcus aureus – review. Folia Microbiol (Praha) 55: 548–558. [DOI] [PubMed] [Google Scholar]
- Moisan H, Brouillette E, Jacob CL, Langlois-Begin P, Michaud S, and Malouin F (2006) Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J Bacteriol 188: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk IR, Shah IM, Xu M, Tan MW, and Foster TJ (2012) Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3(2): e00277–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Ueno K, Uno J, Nagi M, Tanabe K, Aoyama T, et al. (2011) Growth defects resulting from inhibiting ERG20 and RAM2 in Candida glabrata. FEMS Microbiol Lett 317: 27–33. [DOI] [PubMed] [Google Scholar]
- Nielsen LE, Kadavy DR, Rajagopal S, Drijber R, and Nickerson KW (2005) Survey of extreme solvent tolerance in gram-positive cocci: membrane fatty acid changes in Staphylococcus haemolyticus grown in toluene. Appl Environ Microbiol 71: 5171–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norstrom T, Lannergard J, and Hughes D (2007) Genetic and phenotypic identification of fusidic acid-resistant mutants with the small-colony-variant phenotype in Staphylococcus aureus. Antimicrob Agents Chemother 51: 4438–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka B, and Kruk J (2010) Occurrence, biosynthesis and function of isoprenoid quinones. Biochim Biophys Acta 1797: 1587–1605. [DOI] [PubMed] [Google Scholar]
- Okada M (2011) Post-translational isoprenylation of tryptophan. Biosci Biotechnol Biochem 75: 1413–1417. [DOI] [PubMed] [Google Scholar]
- Okada M, Yamaguchi H, Sato I, Tsuji F, Dubnau D, and Sakagami Y (2008) Chemical structure of posttranslational modification with a farnesyl group on tryptophan. Biosci Biotechnol Biochem 72: 914–918. [DOI] [PubMed] [Google Scholar]
- Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, and Gotz F (2005) Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem 280: 32493–32498. [DOI] [PubMed] [Google Scholar]
- Price CT, Jones SC, Amundson KE, and Kwaik YA (2010) Host-mediated post-translational prenylation of novel dot/icm-translocated effectors of legionella pneumophila. Front Microbiol 1: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinicke AT, Hutchinson JL, Magee AI, Mastroeni P, Trowsdale J, and Kelly AP (2005) A Salmonella typhimurium effector protein SifA is modified by host cell prenylation and S-acylation machinery. J Biol Chem 280: 14620–14627. [DOI] [PubMed] [Google Scholar]
- Saiki K, Mogi T, Ogura K, and Anraku Y (1993) In vitro heme O synthesis by the cyoE gene product from Escherichia coli. J Biol Chem 268: 26041–26044. [PubMed] [Google Scholar]
- Shaw LN, Lindholm C, Prajsnar TK, Miller HK, Brown MC, Golonka E, et al. (2008) Identification and characterization of sigma, a novel component of the Staphylococcus aureus stress and virulence responses. PLoS ONE 3: e3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi D, and Niki H (2011) A mutation of ispA that is involved in isoprenoid biogenesis can improve growth of Escherichia coli at low temperatures. Microbiol Immunol 55: 885–888. [DOI] [PubMed] [Google Scholar]
- Sinensky M (1974) Homeoviscous adaptation–a homeo-static process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA 71: 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski WK, Markowska E, Gruszecki WI, and Sielewiesiuk J (1992) Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: a spin-label study. Biochim Biophys Acta 1105: 97–108. [DOI] [PubMed] [Google Scholar]
- Tsuji F, Kobayashi K, Okada M, Yamaguchi H, Ojika M, and Sakagami Y (2011) The geranyl-modified tryptophan residue is crucial for ComXRO-E-2 pheromone biological activity. Bioorg Med Chem Lett 21: 4041–4044. [DOI] [PubMed] [Google Scholar]
- Tsuji F, Ishihara A, Nakagawa A, Okada M, Kitamura S, Kanamaru K, et al. (2012) Lack of the consensus sequence necessary for tryptophan prenylation in the ComX pheromone precursor. Biosci Biotechnol Biochem 76: 1492–1496. [DOI] [PubMed] [Google Scholar]
- Vallon T, Ghanegaonkar S, Vielhauer O, Muller A, Albermann C, Sprenger G, et al. (2008) Quantitative analysis of isoprenoid diphosphate intermediates in recombinant and wild-type Escherichia coli strains. Appl Microbiol Biotechnol 81: 175–182. [DOI] [PubMed] [Google Scholar]
- Weiss A, Ibarra JA, Paoletti J, Carroll RK, and Shaw LN (2014) The delta subunit of RNA polymerase guides promoter selectivity and virulence in Staphylococcus aureus. Infect Immun 82: 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska A, and Subczynski WK (1998) Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim Biophys Acta 1368: 235–246. [DOI] [PubMed] [Google Scholar]
- Wisniewska A, Widomska J, and Subczynski WK (2006) Carotenoid-membrane interactions in liposomes: effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim Pol 53: 475–484. [PubMed] [Google Scholar]
- Wollack JW, Zeliadt NA, Mullen DG, Amundson G, Geier S, Falkum S, et al. (2009) Multifunctional prenylated peptides for live cell analysis. J Am Chem Soc 131: 7293–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, and Casey PJ (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65: 241–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


