ABSTRACT
Background
Some studies suggest that neck dissection (ND) should be avoided in candidates for immunotherapy because lymph nodes are primary sites for immunotherapy activation. Our study investigates ND utilization and associated differences in overall survival (OS) among patients with head and neck cancer (HNC) undergoing adjuvant immunotherapy.
Methods
The 2013–2018 National Cancer Database was retrospectively reviewed for patients with HNC undergoing surgery with curative intent, and adjuvant immunotherapy. Multivariable binary logistic and Cox regression models adjusted for patient demographics, clinicopathologic features, and treatment.
Results
Of 1335 patients satisfying inclusion criteria, 679 (50.9%) patients underwent ND: 94 (13.8%) had pN0, 109 (16.1%) had pN1, 411 (60.5%) had pN2, 60 (8.8%) had pN3, and 5 (0.7%) had pNx classification. On multivariable binary logistic regression, academic treatment facility, cT4, and cN1–3 classification were associated with higher odds of undergoing ND (p < 0.05); salivary, sinonasal, oropharyngeal, hypopharyngeal, and laryngeal primary sites were associated with decreased odds (p < 0.05). Compared with those undergoing neck observation, patients undergoing ND had worse OS (49.4% vs. 61.5%, p < 0.001) on Kaplan–Meier but not multivariable Cox (adjusted hazard ratio [aHR] 1.00, 95% confidence interval [CI] 0.82–1.24, p = 0.968) regression. Compared with adjuvant immunotherapy alone, the addition of radiotherapy (aHR 0.64, 95% CI 0.44–0.93) and chemoradiotherapy (aHR 0.56, 95% CI 0.37–0.86) were associated with higher OS (p < 0.025).
Conclusion
ND was utilized in approximately 51% of patients with HNC undergoing adjuvant immunotherapy. ND was not associated with worse OS, possibly related to the high rate of pN1–3 classification.
Level of Evidence
4
Keywords: head and neck cancer, immunotherapy, National Cancer Database, radiotherapy, survival
Some studies suggest that neck dissection (ND) should be avoided in candidates for immunotherapy because lymph nodes are primary sites for immunotherapy activation. Our study indicates that ND was utilized in approximately 51% of patients with head and neck cancer undergoing adjuvant immunotherapy. ND was not associated with worse overall survival, possibly related to the high rate of pN1–3 classification.

1. Introduction
Clinical evidence of lymph node (LN) metastasis warrants consideration of neck dissection (ND) in head and neck cancer (HNC) management because LN metastasis portends poor locoregional control, recurrence, distant metastasis, and worse survival [1, 2, 3, 4]. Increasing evidence supports consideration of ND for the clinically uninvolved neck because physical examination and imaging modalities lack sufficient sensitivity and accuracy in detecting occult nodal metastasis [5].
The recent advent of immunotherapies targeting cytotoxic T‐lymphocyte antigen 4, programmed cell death ligand‐1, tumor antigens, and cell cycle checkpoints has impacted HNC management, especially in the neoadjuvant setting, early in the disease course [6, 7]. Emerging literature suggests that ND should be avoided in candidates for immunotherapy because tumor‐draining LNs are primary sites for antigen‐presenting cell differentiation, T‐cell priming, and epitope spreading [3, 8, 9, 10, 11, 12, 13, 14]. Interestingly, excising uninvolved LNs is also reported to blunt responses to immunotherapy [15, 16]. Given that immunotherapy is reliant on host immune cells, antigen processing within LNs, and lymphatic vessels, our study of the National Cancer Database (NCDB) investigates ND utilization and associated differences in overall survival (OS) among patients with HNC undergoing adjuvant immunotherapy [17, 18, 19]. To our knowledge, our study is also the first to query a large, nationally representative database exclusively for patients with HNC undergoing immunotherapy.
2. Methods
2.1. Data Source
The NCDB, a national, hospital facility‐based, comprehensive clinical surveillance resource established in 1989, is jointly sponsored by the American Cancer Society and the American College of Surgeons (ACS) Commission on Cancer (CoC), contains data derived from > 1500 CoC‐accredited hospitals, and captures > 70% of all newly diagnosed cancers in the United States annually [20]. The ACS has executed a Business Associate Agreement that includes a data use agreement with each of its CoC‐accredited facilities. This study was deemed exempt from review by the Rutgers New Jersey Medical School Institutional Review Board because of the de‐identified nature of patient data. The ACS and CoC have not verified and are not responsible for the validity of the statistical analysis or conclusions derived herein.
2.2. Inclusion Criteria
A retrospective review of the NCDB was performed for adults with a histologically confirmed diagnosis of HNC between January 2013 and December 2018 and treated with curative intent, including surgery and adjuvant immunotherapy (Figure 1). HNC was identified using the International Classification of Diseases for Oncology, Third Edition (ICD‐O‐3) histology (“8070–8072” for conventional squamous cell carcinoma [SCC] and all remaining histology codes for Other), behavior (“3” for malignant tumors), and topography codes (“C00,” “C02,” “C03,” “C04,” “C05,” and “C06” for oral cavity; “C07” and “C08” for major salivary glands; “C11,” “C30.0,” and “C31” for sinonasal tract; “C01,” “C02.4,” “C05.1,” “C05.2,” “C09,” and “C10” for oropharynx; “C12” and “C13” for hypopharynx; and “C32” for larynx). Patients with a history of prior malignancy; treatment with palliative intent; clinically distant metastasis; treatment without surgery and adjuvant immunotherapy; unknown ND; adjuvant radiotherapy (aRT) volume outside the head and neck; non‐therapeutic aRT dose (< 44 or > 80 Gy); unknown aRT modality, volume, or dose; initiation of aRT > 90 days following surgery; unknown chemotherapy; treatment with hormone therapy; neoadjuvant therapy; unknown sequence of surgery, radiotherapy, and chemotherapy; or unknown vital status or survival were excluded.
FIGURE 1.
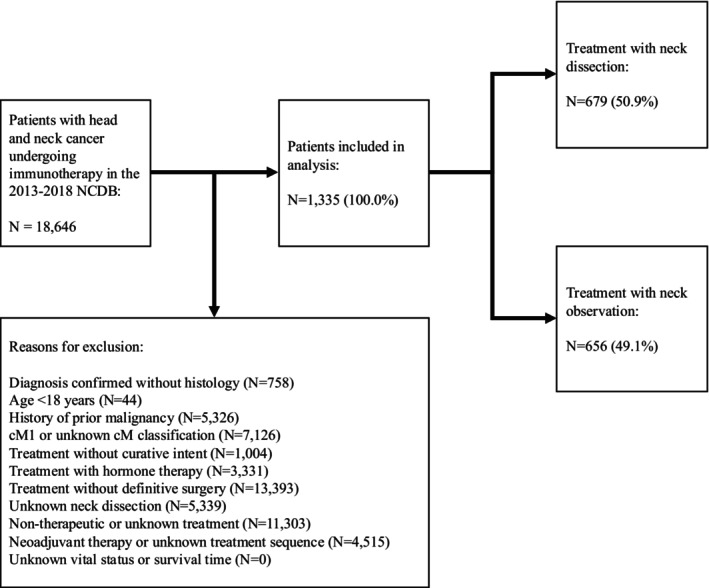
Inclusion criteria. CM, clinical metastasis; NCDB, National Cancer Database.
2.3. Variables
Patient data included age at diagnosis, sex, race, reporting facility type, Charlson‐Deyo comorbidity score (CDCS), history of prior malignancy, histology, primary site, grade, clinical tumor‐nodal‐metastasis, pathologic tumor‐nodal‐metastasis classification, human papillomavirus (HPV), pathologic extranodal extension, lymphovascular invasion (LVI), ND, regional lymph node yield (LNY), surgical margins, surgical length of stay (LOS), 30‐day readmission and 90‐day mortality following surgery, adjuvant therapy, vital status, and survival time. Patients with a CDCS of 0 had no recorded comorbid conditions. Grade was classified as low (i.e., well or moderately differentiated), high (i.e., poorly differentiated, undifferentiated, or anaplastic), or unknown. Surgery included local tumor destruction, local tumor excision, partial removal, total removal, radical removal, and unspecified surgery. ND was defined as the removal and examination of 18 LNs, a validated threshold in HNC (Figure 2) [21, 22, 23, 24]. aRT was defined as the delivery of therapeutic doses (44–80 Gy) of external beam radiation to the head and neck within 90 days of surgery. aCRT was defined as the delivery of any chemotherapy, regardless of the type or number of agents, within 14 days of aRT initiation. The NCDB directly defines immunotherapy (i.e., biologic response modifier) as biologic or chemical agents that alter the immune system or change the host response to tumor cells; starting in 2013, at the beginning of our study period, 6 medications previously classified as chemotherapy were reclassified as immunotherapy: alemtuzumab, bvacizumab, rituximab, trastuzumab, pertuzumab, and cetuxumab. The primary outcome of our study was 5‐year OS. Survival time was calculated as the time from diagnosis to either death or 5 years of follow‐up.
FIGURE 2.
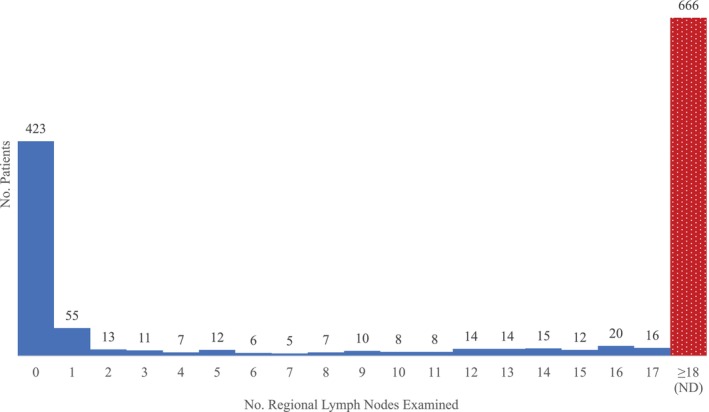
Extent of regional lymph node examination.
2.4. Statistical Analysis
The chi‐square and Mann–Whitney U tests were utilized to compare categorical and continuous variables, respectively. A multivariable binary logistic regression model handling missing data with listwise elimination and adjusting for all significant variables in univariable regression was implemented to identify factors associated with undergoing ND. Kaplan–Meier analysis was performed with the log‐rank test to estimate 5‐year OS. Multivariable Cox proportional hazards regression models handling missing data with listwise elimination included all significant variables from univariable Cox regression. The proportionality of hazards was evaluated using time‐dependent covariables and was not violated. To account for nonlinear behavior of LNY, follow‐up loss, immortal time bias, and the possibly increased radiosensitivity of advanced tumors with poor prognosis, the following 3 sensitivity analyses were conducted: (1) analyzing LNY as a categorical variable, (2) censoring survival > 2 and > 3 years, and (3) sequential landmark survival analysis for patients surviving 6 and 12 months from diagnosis, as previously described [25]. The two‐sided threshold for statistical significance was set at p < 0.05. SPSS version 25 (IBM) and Microsoft Excel (Microsoft) were used for statistical analysis.
3. Results
3.1. Patient Demographics, Clinicopathologic Features, and Treatment
One thousand three hundred and thirty‐five patients satisfied the inclusion criteria. The median (interquartile range [IQR]) patient age at diagnosis was 63 (56–70) years. A high proportion of patients were male (N = 991, 74.2%), of White race (N = 1186, 89.2%), without comorbidities by the CDCS (N = 1073, 80.4%), and had conventional SCC (N = 1122, 84.0%) of the oropharynx (N = 583, 43.7%) classified as low‐grade (N = 516, 38.7%), cT2 (N = 446, 33.4%), and cN2 (N = 588, 44.0%) (Table 1). Most patients had negative surgical margins (N = 731, 62.5%) and underwent ND (N = 679, 50.9%). Immunotherapy + radiotherapy (N = 939, 70.3%) was the most common adjuvant therapy combination, followed by immunotherapy + chemoradiotherapy (N = 282, 21.1%), immunotherapy alone (N = 74, 5.5%), and immunotherapy + chemotherapy (N = 40, 3.0%). The median (IQR) time from surgery to adjuvant chemotherapy, aRT, and adjuvant immunotherapy was 47 (36–60), 47 (35–58), and 47 (34–63) days, respectively. The median (IQR) aRT dose was 60 (58–66) Gy. Following surgery, patients undergoing ND and neck observation had similar 30‐day readmission (N = 38 [5.8%] vs. N = 31 [4.9%], p = 0.467) and 90‐day mortality (N = 4 [0.6%] vs. N = 4 [0.6%], p = 0.959).
TABLE 1.
Patient demographics, clinicopathologic features, and adjuvant therapy by neck dissection, n (%).
| Neck observation | Neck dissection | p | Total | |
|---|---|---|---|---|
| No. | 656 (49.1) | 679 (50.9) | — | 1335 |
| Age at diagnosis, median years (IQR) | 63 (56–71) | 62 (55–70) | 0.139 | 63 (56–70) |
| Sex | ||||
| Male | 478 (72.9) | 513 (75.6) | 0.262 | 991 (74.2) |
| Female | 178 (27.1) | 166 (24.4) | 344 (25.8) | |
| Race | ||||
| White | 588 (90) | 598 (88.5) | 0.586 | 1186 (89.2) |
| Black | 44 (6.7) | 50 (7.4) | 94 (7.1) | |
| Other | 21 (3.2) | 28 (4.1) | 49 (3.7) | |
| Reporting facility type | ||||
| Academic/research | 234 (36.4) | 363 (54.8) | < 0.001 | 597 (45.7) |
| Non‐academic/research | 409 (63.6) | 299 (45.2) | 708 (54.3) | |
| CDCS | ||||
| 0 | 543 (82.8) | 530 (78.1) | 0.093 | 1073 (80.4) |
| 1 | 74 (11.3) | 96 (14.1) | 170 (12.7) | |
| 2 | 39 (5.9) | 53 (7.8) | 92 (6.9) | |
| Histology | ||||
| Conventional SCC | 528 (80.5) | 594 (87.5) | < 0.001 | 1122 (84.0) |
| Other | 128 (19.5) | 85 (12.5) | 213 (16.0) | |
| Primary site | ||||
| Oral cavity | 97 (14.8) | 293 (43.2) | < 0.001 | 390 (29.2) |
| Major salivary glands | 35 (5.3) | 57 (8.4) | 92 (6.9) | |
| Sinonasal tract | 69 (10.5) | 10 (1.5) | 79 (5.9) | |
| Oropharynx | 362 (55.2) | 221 (32.5) | 583 (43.7) | |
| Hypopharynx | 19 (2.9) | 11 (1.6) | 30 (2.2) | |
| Larynx | 74 (11.3) | 87 (12.8) | 161 (12.1) | |
| Grade | ||||
| Low | 229 (34.9) | 287 (42.3) | 0.013 | 516 (38.7) |
| High | 219 (33.4) | 215 (31.7) | 434 (32.5) | |
| Unknown | 208 (31.7) | 177 (26.1) | 385 (28.8) | |
| cT classification | ||||
| 1 | 126 (19.2) | 99 (14.6) | < 0.001 | 225 (16.9) |
| 2 | 230 (35.1) | 216 (31.8) | 446 (33.4) | |
| 3 | 124 (18.9) | 92 (13.5) | 216 (16.2) | |
| 4 | 80 (12.2) | 173 (25.5) | 253 (19.0) | |
| x | 96 (14.6) | 99 (14.6) | 195 (14.6) | |
| cN classification | ||||
| 0 | 223 (34) | 188 (27.7) | 0.007 | 411 (30.8) |
| 1 | 137 (20.9) | 132 (19.4) | 269 (20.1) | |
| 2 | 273 (41.6) | 315 (46.4) | 588 (44.0) | |
| 3 | 12 (1.8) | 15 (2.2) | 411 (30.8) | |
| x | 11 (1.7) | 29 (4.3) | 269 (20.1) | |
| pENE | ||||
| No | — | 309 (49.0) | < 0.001 | 451 (52.9) |
| Yes | — | 321 (51.0) | 401 (47.1) | |
| LVI | ||||
| No | 279 (75.6) | 338 (55.4) | < 0.001 | 617 (63.0) |
| Yes | 90 (24.4) | 272 (44.6) | 362 (37.0) | |
| Surgical margins | ||||
| Negative | 236 (45.9) | 495 (75.6) | < 0.001 | 731 (62.5) |
| Positive | 278 (54.1) | 160 (24.4) | 438 (37.5) | |
| No. regional lymph nodes examined, median (IQR) | 62 (55–70) | 0 (0–5) | < 0.001 | 20 (0–38) |
| Surgical outcomes | ||||
| LOS, median days (IQR) | 0 (0–5) | 36 (27–52) | < 0.001 | 2 (0–7) |
| 30‐day readmission | 31 (4.9) | 38 (5.8) | 0.467 | 69 (5.3) |
| 90‐day mortality | 4 (0.6) | 4 (0.6) | 0.959 | 8 (0.6) |
| Adjuvant therapy | ||||
| Immunotherapy | 37 (5.6) | 37 (5.4) | 0.049 | 74 (5.5) |
| Immunotherapy + chemotherapy | 14 (2.1) | 26 (3.8) | 40 (3.0) | |
| Immunotherapy + RT | 481 (73.3) | 458 (67.5) | 939 (70.3) | |
| Immunotherapy + CRT | 124 (18.9) | 158 (23.3) | 282 (21.1) | |
| Adjuvant RT | ||||
| Duration, median days (IQR) | 49 (43–52) | 45 (42–49) | < 0.001 | 46 (43–51) |
| Dose, median Gy (IQR) | 66 (56–70) | 60 (60–66) | < 0.001 | 60 (58–66) |
| Adjuvant therapy timing, median days (IQR) | ||||
| Diagnosis to surgery | 16 (0–34) | 35 (22–50) | < 0.001 | 28 (10–44) |
| Surgery to chemotherapy | 42 (33–56) | 50 (40–62) | < 0.001 | 47 (36–60) |
| Surgery to radiotherapy | 41 (33–55) | 50 (41–62) | < 0.001 | 47 (35–58) |
| Surgery to immunotherapy | 40 (28–59) | 50 (40–65) | < 0.001 | 47 (34–63) |
Note: Bold values are statistically significant (p < 0.05).
Abbreviations: CDCS, Charlson‐Deyo comorbidity score; CRT, chemoradiotherapy; cTN, clinical tumor‐nodal; IQR, interquartile range; LOS, length of stay; LVI, lymphovascular invasion; pENE, pathologic extranodal extension; RT, radiotherapy.
Categorizing age at diagnosis as 18–49 years (N = 137, 10.3%), 50–59 years (N = 362, 27.1%), 60–69 years (N = 460, 34.5%), 70–79 years (N = 285, 21.3%), and 80 years (N = 91, 6.8%), utilization of ND and immunotherapy + chemoradiotherapy was highest among age 18–49 years (Figure 3).
FIGURE 3.
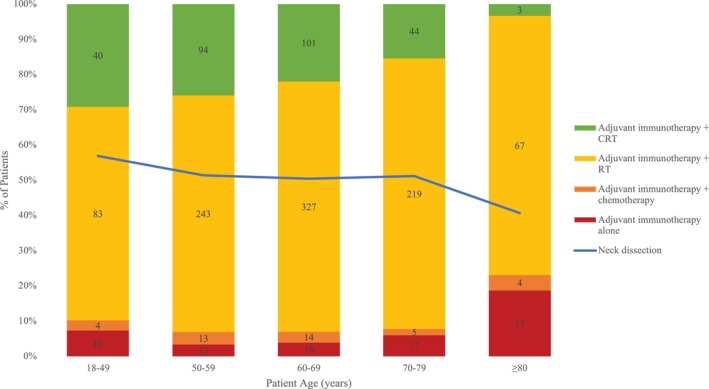
Utilization of neck dissection and adjuvant therapy by age. CRT, chemoradiotherapy; ND, neck dissection; RT, radiotherapy.
On multivariable binary logistic regression, academic treatment facility, cT4, and cN1–3 classification were associated with higher odds of undergoing ND (p < 0.05); salivary, sinonasal, oropharyngeal, hypopharyngeal, and laryngeal primary sites were associated with decreased odds (p < 0.05) (Table 2).
TABLE 2.
Kaplan–Meier analysis of 5‐year OS (%) by neck dissection with log‐rank comparisons within strata.
| Neck observation | Neck dissection | p | Total | |
|---|---|---|---|---|
| Overall | 61.5 | 49.4 | < 0.001 | 55.2 |
| Histology | ||||
| Conventional SCC | 61.8 | 48.9 | < 0.001 | 54.9 |
| Other | 60.0 | 52.4 | 0.320 | 56.8 |
| Primary site | ||||
| Oral cavity | 34.2 | 39.3 | 0.695 | 38.1 |
| Major salivary glands | 51.7 | 49.5 | 0.908 | 50.3 |
| Sinonasal tract | 52.6 | 26.3 | 0.170 | 48.3 |
| Oropharynx | 78.2 | 76.3 | 0.811 | 77.5 |
| Hypopharynx | 31.3 | — | 0.892 | 25.5 |
| Larynx | 42.9 | 30.6 | 0.122 | 36.1 |
| Grade | ||||
| Low | 56.8 | 46.2 | 0.027 | 50.8 |
| High | 68.0 | 53.1 | 0.003 | 60.3 |
| Unknown | 59.9 | 50.2 | 0.092 | 55.3 |
| cT classification | ||||
| 1 | 76.9 | 63.5 | 0.037 | 70.9 |
| 2 | 66.2 | 60.9 | 0.417 | 63.6 |
| 3 | 49.0 | 28.5 | 0.003 | 39.5 |
| 4 | 32.3 | 39.1 | 0.260 | 37.0 |
| x | 74.1 | 53.3 | 0.005 | 63.3 |
| cN classification | ||||
| 0 | 52.7 | 48.5 | 0.565 | 50.8 |
| 1 | 73.2 | 53.2 | 0.003 | 63.0 |
| 2 | 63.1 | 49.0 | < 0.001 | 55.4 |
| 3 | 52.9 | 45.5 | 0.791 | 48.7 |
| x | 76.5 | 44.7 | 0.125 | 53.1 |
| pENE | ||||
| No | 65.0 | 55.3 | 0.066 | 58.2 |
| Yes | 50.4 | 43.9 | 0.180 | 45.1 |
| LVI | ||||
| No | 64.3 | 53.8 | 0.017 | 58.4 |
| Yes | 45.5 | 41.5 | 0.392 | 42.5 |
| Surgical margins | ||||
| Negative | 63.4 | 51.1 | 0.002 | 55.0 |
| Positive | 61.3 | 41.9 | 0.001 | 53.9 |
| Adjuvant therapy | ||||
| Immunotherapy | 45.5 | 27.9 | 0.054 | 36.2 |
| Immunotherapy + chemotherapy | 33.3 | 44.2 | 0.565 | 40.3 |
| Immunotherapy + RT | 62.8 | 49.6 | < 0.001 | 56.2 |
| Immunotherapy + CRT | 65.0 | 55.0 | 0.050 | 59.2 |
Note: Bold values are statistically significant (p < 0.05).
Abbreviations: CRT, chemoradiotherapy; cTN, clinical tumor‐nodal; LVI, lymphovascular invasion; OS, overall survival; pENE, pathologic extranodal extension; RT, radiotherapy; SCC, squamous cell carcinoma.
3.2. 5‐Year OS
On Kaplan–Meier analysis, patients undergoing ND had worse 5‐year OS than those undergoing neck observation (49.4% vs. 61.5%, p < 0.001) (Figure 4, Table 3). The 5‐year OS for patients undergoing adjuvant immunotherapy alone, adjuvant immunotherapy + chemotherapy, adjuvant immunotherapy + radiotherapy, and adjuvant immunotherapy + chemoradiotherapy was 36.2%, 40.3%, 56.2%, and 59.2%, respectively (p < 0.001).
FIGURE 4.
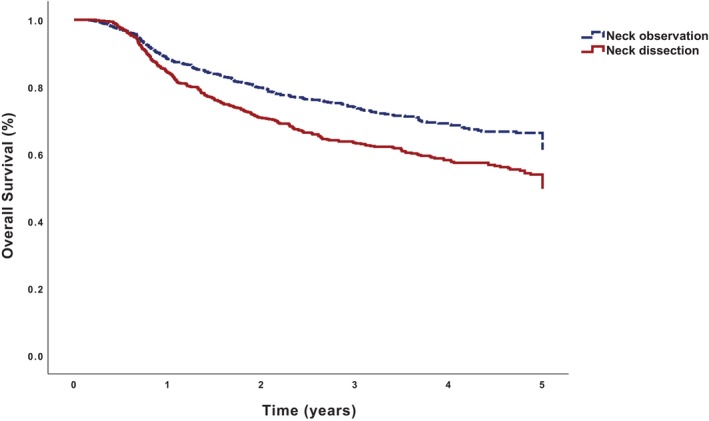
Kaplan–Meier analysis of 5‐year overall survival for 656 patients undergoing neck observation and 679 patients undergoing neck dissection.
TABLE 3.
Univariable and multivariable binary logistic regression predicting preoperative factors associated with undergoing ND.
| OR (95% CI) | p | aOR a (95% CI) | p | |
|---|---|---|---|---|
| Age at diagnosis (years) | 0.99 (0.98–1.00) | 0.099 | ||
| Sex | ||||
| Male | Ref | |||
| Female | 0.87 (0.68–1.11) | 0.262 | ||
| Race | ||||
| White | Ref | |||
| Black | 1.12 (0.73–1.70) | 0.605 | ||
| Other | 1.31 (0.74–2.33) | 0.358 | ||
| Reporting facility type | ||||
| Academic | 2.12 (1.70–2.65) | < 0.001 | 2.13 (1.66–2.73) | < 0.001 |
| Non‐academic | Ref | Ref | ||
| CDCS | ||||
| 0 | Ref | |||
| 1 | 1.33 (0.96–1.84) | 0.087 | ||
| 2 | 1.39 (0.91–2.14) | 0.132 | ||
| Histology | ||||
| Conventional SCC | Ref | Ref | ||
| Other | 0.59 (0.44–0.80) | 0.001 | 0.82 (0.55–1.23) | 0.344 |
| Primary site | ||||
| Oral cavity | Ref | Ref | ||
| Major salivary glands | 0.54 (0.33–0.87) | 0.012 | 0.54 (0.31–0.94) | 0.029 |
| Sinonasal tract | 0.05 (0.02–0.10) | < 0.001 | 0.05 (0.02–0.11) | < 0.001 |
| Oropharynx | 0.20 (0.15–0.27) | < 0.001 | 0.18 (0.12–0.25) | < 0.001 |
| Hypopharynx | 0.19 (0.09–0.42) | < 0.001 | 0.15 (0.06–0.35) | < 0.001 |
| Larynx | 0.39 (0.26–0.57) | < 0.001 | 0.34 (0.22–0.52) | < 0.001 |
| Grade | ||||
| Low | Ref | Ref | ||
| High | 0.78 (0.61–1.01) | 0.062 | 1.16 (0.86–1.57) | 0.335 |
| Unknown | 0.68 (0.52–0.89) | 0.004 | 1.07 (0.78–1.48) | 0.673 |
| cT classification | ||||
| 1 | Ref | Ref | ||
| 2 | 1.20 (0.87–1.65) | 0.278 | 1.09 (0.76–1.55) | 0.638 |
| 3 | 0.94 (0.65–1.38) | 0.766 | 0.90 (0.58–1.41) | 0.658 |
| 4 | 2.75 (1.89–4.00) | < 0.001 | 2.15 (1.35–3.41) | 0.001 |
| x | 1.31 (0.89–1.93) | 0.166 | 1.22 (0.80–1.87) | 0.356 |
| cN classification | ||||
| 0 | Ref | Ref | ||
| 1 | 1.14 (0.84–1.55) | 0.395 | 1.78 (1.22–2.58) | 0.003 |
| 2 | 1.37 (1.06–1.76) | 0.015 | 2.13 (1.54–2.96) | < 0.001 |
| 3 | 1.48 (0.68–3.25) | 0.324 | 2.59 (1.02–6.62) | 0.046 |
| x | 3.13 (1.52–6.43) | 0.002 | 4.93 (2.21–11.03) | < 0.001 |
Note: Bold values are statistically significant (p < 0.05).
Abbreviations: aOR, adjusted odds ratio; CDCS, Charlson‐Deyo comorbidity score; CI, confidence interval; cTN, clinical tumor‐nodal; OR, odds ratio; Ref, reference; SCC, squamous cell carcinoma.
N = 1305; total number of neck dissection events: 764.
On multivariable Cox regression, age at diagnosis, cT3–4, and cN2–3 classification were associated with worse OS (p < 0.05); salivary, oropharyngeal primary sites, immunotherapy + radiotherapy (adjusted hazard ratio [aHR] 0.64, 95% confidence interval [CI] 0.44–0.93), and immunotherapy + chemoradiotherapy (aHR 0.56, 95% CI 0.37–0.86) were associated with higher OS (p < 0.025) (Table 4). ND (aHR 1.00, 95% CI 0.82–1.24, p = 0.968) was not associated with OS.
TABLE 4.
Univariable and multivariable Cox proportional hazards regression models.
| HR (95% CI) | p | aHR a (95% CI) | p | |
|---|---|---|---|---|
| Age at diagnosis (years) | 1.03 (1.02–1.04) | < 0.001 | 1.02 (1.01–1.03) | < 0.001 |
| Sex | ||||
| Male | Ref | |||
| Female | 1.14 (0.94–1.40) | 0.187 | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.48 (1.07–2.03) | 0.016 | 1.03 (0.74–1.43) | 0.867 |
| Other | 1.92 (1.28–2.87) | 0.002 | 1.51 (1.00–2.28) | 0.051 |
| Reporting facility type | ||||
| Academic | 0.96 (0.80–1.15) | 0.682 | ||
| Non‐academic | Ref | |||
| CDCS | ||||
| 0 | Ref | Ref | ||
| 1 | 1.09 (0.84–1.40) | 0.516 | 0.97 (0.75–1.25) | 0.801 |
| 2 | 1.43 (1.04–1.95) | 0.026 | 1.25 (0.91–1.73) | 0.171 |
| Histology | ||||
| Conventional SCC | Ref | |||
| Other | 0.93 (0.73–1.19) | 0.558 | ||
| Primary site | ||||
| Oral cavity | Ref | Ref | ||
| Major salivary glands | 0.66 (0.47–0.94) | 0.020 | 0.61 (0.42–0.88) | 0.008 |
| Sinonasal tract | 0.73 (0.50–1.06) | 0.096 | 0.70 (0.46–1.09) | 0.113 |
| Oropharynx | 0.26 (0.20–0.33) | < 0.001 | 0.29 (0.22–0.39) | < 0.001 |
| Hypopharynx | 1.43 (0.89–2.29) | 0.135 | 1.40 (0.86–2.29) | 0.180 |
| Larynx | 1.05 (0.82–1.35) | 0.702 | 0.91 (0.70–1.19) | 0.510 |
| Grade | ||||
| Low | Ref | Ref | ||
| High | 0.74 (0.60–0.92) | 0.006 | 0.97 (0.78–1.22) | 0.801 |
| Unknown | 0.85 (0.68–1.07) | 0.164 | 0.85 (0.66–1.09) | 0.203 |
| cT classification | ||||
| 1 | Ref | Ref | ||
| 2 | 1.42 (1.03–1.98) | 0.035 | 1.14 (0.82–1.60) | 0.427 |
| 3 | 3.01 (2.15–4.21) | < 0.001 | 1.98 (1.39–2.82) | < 0.001 |
| 4 | 3.38 (2.44–4.69) | < 0.001 | 1.74 (1.23–2.48) | 0.002 |
| x | 1.39 (0.95–2.04) | 0.092 | 1.39 (0.93–2.08) | 0.105 |
| cN classification | ||||
| 0 | Ref | Ref | ||
| 1 | 0.65 (0.49–0.85) | 0.002 | 1.03 (0.78–1.37) | 0.830 |
| 2 | 0.87 (0.71–1.07) | 0.178 | 1.59 (1.26–2.00) | < 0.001 |
| 3 | 1.05 (0.57–1.93) | 0.875 | 1.99 (1.06–3.72) | 0.032 |
| x | 1.04 (0.63–1.71) | 0.889 | 1.28 (0.75–2.18) | 0.370 |
| Neck dissection | ||||
| No | Ref | Ref | ||
| Yes | 1.45 (1.21–1.74) | < 0.001 | 1.00 (0.82–1.23) | 0.968 |
| Surgical margins | ||||
| Negative | Ref | |||
| Positive | 1.02 (0.84–1.24) | 0.858 | ||
| Adjuvant therapy | ||||
| Immunotherapy | Ref | Ref | ||
| Immunotherapy + chemotherapy | 0.93 (0.54–1.59) | 0.792 | 1.00 (0.57–1.77) | 0.986 |
| Immunotherapy + RT | 0.48 (0.34–0.67) | < 0.001 | 0.64 (0.44–0.93) | 0.018 |
| Immunotherapy + CRT | 0.44 (0.30–0.65) | < 0.001 | 0.56 (0.37–0.86) | 0.007 |
Note: Bold values are statistically significant (p < 0.05).
Abbreviations: aHR, adjusted hazard ratio; CDCS, Charlson‐Deyo comorbidity score; CI, confidence interval; CRT, chemoradiotherapy; cTN, clinical tumor‐nodal; HR, hazard ratio; OS, overall survival; Ref, reference; RT, radiotherapy; SCC, squamous cell carcinoma.
N = 1329; total number of uncensored death events: 482.
3.3. pTN Classification
Among 656 patients undergoing neck observation, 166 (25.3%) had pT1, 151 (23.0%) had pT2, 86 (13.1%) had pT3, 83 (12.7%) had pT4, and 170 (25.9%) had pTx classification.
Among 679 patients undergoing ND, 119 (17.5%) had pT1, 199 (29.3%) had pT2, 124 (18.3%) had pT3, 223 (32.8%) had pT4, and 14 (2.1%) had pTx classification. Among these patients, 94 (13.8%) had pN0, 109 (16.1%) had pN1, 411 (60.5%) had pN2, 60 (8.8%) had pN3, and 5 (0.7%) had pNx classification.
3.4. Secondary Outcomes
Compared with neck observation, ND was associated with longer LOS (4.00 marginal days, 95% CI 3.26–4.75 days, p < 0.001) but similar 90‐day mortality (adjusted odds ratio 1.29, 95% CI 0.75–2.23, p = 0.355) on multivariable binary logistic regression adjusting for patient demographics and clinicopathologic features.
3.5. HPV and Oropharyngeal Cancer
Four hundred and seventy‐three patients with oropharyngeal cancer had known HPV status: 71 (15.0%) were HPV negative and 402 (85.0%) were HPV positive.
Among HPV‐negative patients, 41 underwent neck observation and 30 underwent ND; 5‐year OS was 67.7% and 51.0%, respectively, on Kaplan–Meier analysis (p = 0.181). ND was not associated with OS on multivariable Cox regression (aHR 2.25, 95% CI 0.68–7.49, p = 0.186).
Among HPV‐positive patients, 258 underwent neck observation and 144 underwent ND; 5‐year OS was 80.9% and 87.9%, respectively, on Kaplan–Meier analysis (p = 0.092). ND was not associated with OS on multivariable Cox regression (aHR 0.53, 95% CI 0.27–1.01, p = 0.054).
3.6. LNY
Instead of binarizing LNY at 18 LNs to define ND, LNY was categorized as 0 (N = 423, 32.0%), 1–5 (N = 98, 7.4%), 6–10 (N = 36, 2.7%), 11–15 (N = 63, 4.8%), 16–20 (N = 85, 6.4%), 21–25 (N = 91, 6.9%), 26–30 (N = 94, 7.1%), 31–35 (N = 86, 6.5%), 36–40 (N = 64, 4.8%), and 40 (N = 282, 21.3%) LNs to identify a possible inflection point; 5‐year OS was 61.6%, 71.2%, 64.3%, 44.6%, 53.4%, 52.4%, 48.6%, 55.6%, 53.4%, and 46.3%, respectively, on Kaplan–Meier analysis (p = 0.001). Compared with LNY of 0, LNY of 1–5 (aHR 0.68, 95% CI 0.42–1.10, p = 0.119), 6–10 (aHR 0.90, 95% CI 0.46–1.74, p = 0.750), 11–15 (aHR 1.29, 95% CI 0.83–1.99, p = 0.253), 16–20 (aHR 0.99, 95% CI 0.65–1.49, p = 0.957), 21–25 (aHR 1.04, 95% CI 0.69–1.55, p = 0.864), 26–30 (aHR 1.06, 95% CI 0.72–1.56, p = 0.780), 31–35 (aHR 0.92, 95% CI 0.61–1.40, p = 0.710), 36–40 (aHR 0.89, 95% CI 0.56–1.40, p = 0.615), and 40 (aHR 0.93, 95% CI 0.70–1.24, p = 0.622) LNs were all not associated with OS on multivariable Cox regression.
3.7. 2‐Year and 3‐Year OS
On Kaplan–Meier analysis, patients undergoing ND had worse 2‐year (70.5% vs. 79.5%) and 3‐year OS (61.9% vs. 72.6%) than those undergoing neck observation (p < 0.001). However, on multivariable Cox regression, ND was not associated with worse 2‐year (aHR 1.00, 95% CI 0.82–1.24, p = 0.968) or 3‐year OS (aHR 1.00, 95% CI 0.82–1.24, p = 0.968) than neck observation.
3.8. 6‐Month and 12‐Month Landmark Survival Analysis
41 (3.2%) and 208 (15.6%) patients had survival times < 6 and < 12 months, respectively. After censoring survival times < 6 months, ND was associated with worse 5‐year OS on Kaplan–Meier (50.8% vs. 63.8%) but not multivariable Cox regression (aHR 1.03, 95% CI 0.83–1.27, p = 0.816). After censoring survival times < 12 months, ND was also associated with worse 5‐year OS on Kaplan–Meier (60.7% vs. 72.2%, p < 0.001) but not multivariable Cox regression (aHR 1.10, 95% CI 0.85–1.44, p = 0.471).
4. Discussion
Clinical evidence of LN metastasis warrants consideration of ND when pursuing surgical management of HNC. Several randomized controlled trials (RCTs) have precipitated hope that immunotherapy may reduce LN metastasis, but response rates remain poor, especially in recurrent or distantly metastatic disease [3, 26, 27]. Increasing evidence suggests that LNs are important sites for immunotherapy activation, raising questions regarding the potential unintended adverse effects of ND [19, 28]. Our study investigating ND among patients with HNC undergoing adjuvant immunotherapy suggests that ND is frequently utilized and is not associated with worse OS.
A high proportion of patients (51%) in our cohort underwent ND. Academic treatment facility, cT4, and cN1–3 classification were associated with undergoing ND. Increased utilization of ND at academic facilities may be driven by access to inpatient hospital resources, tumor boards, and multidisciplinary specialists. Salivary, sinonasal, oropharyngeal, hypopharyngeal, and laryngeal primary sites were associated with undergoing neck observation. Importantly, the increased prevalence of multimorbidity, malnutrition, and poor performance status in elderly patients may contraindicate surgical interventions such as ND, but our study did not detect associations between older age, higher CDCS, and undergoing neck observation. Many mucosal head and neck primary sites have a high propensity for LN metastasis and warrant aggressive neck management, such as elective ND in the setting of cN0 disease to prophylactically remove clinically negative LNs that have a significant probability of harboring occult metastasis [29].
ND was not associated with worse OS after adjusting for patient demographics, clinicopathologic features, and treatment; sensitivity analysis categorizing LNY in increments of 5 LNs did not associate more extensive nodal dissections with OS. Anecdotal experiences of surgeons associating ND with worse survival may be driven by differences in baseline clinicopathologic features, as evidenced by patients in our cohort undergoing ND also having a higher cTN classification. These findings conflict with recent studies suggesting the possible benefit of LN preservation in improving immunotherapy response. LNs are primary sites for (1) migration of lymphocytes, neutrophils, and antigen‐presenting cells such as dendritic cells, and (2) the filtration of antigenic and infectious substances from peripheral tissues, and therefore have a unique role in facilitating the acquisition of adaptive anti‐tumor immunity [14, 16, 19, 30, 31, 32, 33, 34, 35, 36, 37, 38]. Patent lymphatic vessels, however, permit drainage and colonization of tumor cells in LNs, forming LN metastasis [14]. As tumor colonization progresses, immuno‐stimulation in LNs tends to shift to immunosuppression, contributing to tumor immune escape, a dynamic editing effect characterized by defective antigen presentation, T‐cell regulation, depleted T‐cell potency, and uncontrolled cell proliferation due to tumor‐specific immune tolerance [19, 39, 40, 41, 42, 43]. The existence of an immunosuppressed cellular niche in metastatic LNs is supported by studies demonstrating worse survival among patients found to have LN metastasis despite neoadjuvant immunotherapy [44]. Metastatic LNs likely exert immunosuppressive effects and have less value for preservation because unresected LN metastasis accelerates disease progression despite aggressive adjuvant therapy.
Compared with physical examination and imaging, pathologic evaluation with ND provides the most accurate diagnosis of LN metastasis [45]. Sparing patients with a low probability of LN metastasis from undergoing ND could theoretically preserve quality of life and optimize immunotherapy response. For example, ND is associated with increased medical costs, operative time, and aesthetic and functional morbidities such as scarring, shoulder weakness, lymphedema, and impaired sensation [46]. Another consideration for neck management is sentinel LN biopsy, which preserves the majority of the neck lymphatics while offering accurate pathologic staging. Of note, research on cancer phylogenies suggests that LN and distant metastasis may have independent clonal origins, and lymphatic vessels may not be the direct pathway for distant metastasis [47, 48]. Considering the important role of LNs in immunotherapy response, arguments favoring their preservation have become compelling [14]. Techniques capable of accurately diagnosing LN metastasis without extensive nodal dissections, such as those involving deep learning artificial intelligence, liquid biopsy of blood for tumor biomarkers, and nanoparticle lymphatic imaging, are currently under development to better identify candidates for neck observation [14, 49, 50, 51, 52].
Among the 4 adjuvant therapy combinations analyzed in our study, the addition of radiotherapy was associated with the highest OS. Radiotherapy is the most utilized adjuvant therapy in HNC because of its established and predictable safety profile, widespread availability, and potentially immunostimulatory effects by increased antigen presentation occasionally progressing to an abscopal effect [36]. Several RCTs investigating immunoradiotherapy combinations, however, have failed to demonstrate a therapeutic benefit compared with either modality alone, possibly reflecting suboptimal study design, choice of outcomes, and administration of radiotherapy according to standard schedules and target volumes [34, 35, 36, 53]; an ongoing RCT is expected to provide more conclusive evidence [54]. One important consideration in HNC is LN‐sparing aRT, which is associated with improved anti‐tumor responses both locally within the irradiated tumor and tumor‐draining LNs, and systemically within distant LNs and secondary tumors [18, 34, 35, 53, 54, 55, 56, 57, 58]. Studies documenting locoregional recurrence despite LN‐sparing aRT confirm the function of tumor‐draining LNs as supportive niches for cancer dissemination; for these patients, a viable strategy may involve delaying LN irradiation and ND for several weeks until immunotherapy can establish adaptive anti‐tumor immunity, and then initiating comprehensive adjuvant therapy to eradicate persistent malignancy and prevent clinicopathologic upstaging [35]. Another viable strategy is initiating immunotherapy before surgery, when the lymphatics are intact, such as in Keynote 689. Although adjuvant immunotherapy + chemotherapy has been associated with remission in several cancers, the addition of chemotherapy without radiotherapy did not improve OS in our cohort [59].
Older age, cT3–4, and cN2–3 classification were associated with worse OS, aligning with existent literature [60]. Major salivary gland and oropharyngeal primary sites were associated with higher OS, possibly related to changing demographics increasing the incidence of HPV‐positive oropharyngeal cancer, which tends to occur in younger, healthier patients and portends a more favorable response to treatment, as our data reflect [61, 62, 63, 64, 65, 66].
Limitations inherent in the retrospective study of the NCDB include the possibility of inaccurate histologic diagnosis and variable miscoding. The NCDB does not report specific medical comorbidities, tobacco use, tumor mutations, history of neck irradiation, imaging studies, depth of invasion, extent of LVI, metastatic volume, intraoperative tumor spillage, close margins, PNI, multidisciplinary tumor board recommendations, quality of life, locoregional recurrence, and disease‐free survival. The NCDB also does not report (1) whether surgical margins were obtained from the specimen or tumor bed, (2) whether radiotherapy only targeted tumor‐draining LNs or spared uninvolved LNs, (3) the number and type of immunotherapy agents administered, (4) whether immunotherapy and radiotherapy were administered concurrently or sequentially, and (5) treatment delays, interruptions, and extent of completion. Regarding treatment sequence, the NCDB does not differentiate between neoadjuvant systemic therapies (i.e., patients undergoing neoadjuvant immunotherapy cannot be distinctly identified from those undergoing neoadjuvant chemotherapy), limiting our analysis to the adjuvant setting, despite increasing evidence suggesting that immunotherapy has the most significant survival benefit if administered early in the disease course before surgery, radiotherapy, or pathologic evaluation of nodal metastasis can be attempted [6]. Nevertheless, ND is possibly less associated with neoadjuvant immunotherapy response than adjuvant immunotherapy response, especially if sufficient time elapses between neoadjuvant immunotherapy administration and surgery, supporting our exclusion of patients undergoing neoadjuvant systemic therapy. National Comprehensive Cancer Network recommendations for HNC do not include adjuvant immunotherapy, representing a selection bias. Many patients undergoing immunotherapy have distant metastasis, but these patients are typically not candidates for surgery with curative intent and were therefore excluded, representing another selection bias. In fact, < 10% of approximately 19,000 potential patients on the initial database query satisfied our strict inclusion criteria, because a high proportion of patients had unknown ND, treatment, or treatment sequence; our cohort, although large and drawn from a national network of CoC‐accredited facilities, may not be representative of all patients with HNC undergoing immunotherapy (Figure 1). A minority of patients underwent surgery without ND and without aRT (N = 51, 7.7%); our study is therefore unable to draw conclusions regarding immunotherapy in patients with intact lymphatics because ND and radiotherapy may ablate these structures.
5. Conclusion
Approximately 51% of patients with HNC undergoing surgery + adjuvant immunotherapy also underwent ND. Academic treatment facility, cT4, and cN1–3 classification were associated with higher odds of undergoing ND; salivary, sinonasal, oropharyngeal, hypopharyngeal, and laryngeal primary sites were associated with decreased odds. ND was not associated with worse OS, possibly related to the high rate of pathologic nodal metastasis and previous reports of tumor progression shifting LNs from a beneficial immune‐stimulatory to a harmful immunosuppressive phenotype. Although speculative, the benefit of neck observation in improving immunotherapy response likely occurs only if LNs are free of disease, but accurately identifying LN metastasis without ND remains challenging despite advances in diagnostic technologies. ND should be offered whenever indicated because the benefits of removing possibly metastatic LNs likely outweigh the theoretical risk of reducing the efficacy of adjuvant immunotherapy. A well‐designed RCT may elucidate optimal neck management among patients with HNC undergoing immunotherapy and which patients can safely avoid ND without compromising survival.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1. Manukian G., Bar‐Ad V., Lu B., Argiris A., and Johnson J. M., “Combining Radiation and Immune Checkpoint Blockade in the Treatment of Head and Neck Squamous Cell Carcinoma,” Frontiers in Oncology 9 (2019): 122, 10.3389/fonc.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfister D. G., Spencer S., Adelstein D., et al., “Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology,” Journal of the National Comprehensive Cancer Network 18, no. 7 (2020): 873–898, 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 3. McBride S., Sherman E., Tsai C. J., et al., “Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma,” Journal of Clinical Oncology 39, no. 1 (2021): 30–37, 10.1200/JCO.20.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad A., Carey R. M., Panara K., et al., “Nodal Metastasis in Surgically Treated Laryngeal Squamous Cell Carcinoma,” Head & Neck 45, no. 9 (2023): 2303–2312, 10.1002/hed.27437. [DOI] [PubMed] [Google Scholar]
- 5. Hui C., Chau B., Gan G., Stokes W., Karam S. D., and Amini A., “Overcoming Resistance to Immunotherapy in Head and Neck Cancer Using Radiation: A Review,” Frontiers in Oncology 11 (2021): 592319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoenfeld J. D., Hanna G. J., Jo V. Y., et al., “Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma,” JAMA Oncology 6, no. 10 (2020): 1563–1570, 10.1001/jamaoncol.2020.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salem A. F., Chen M. M., Williams M. D., et al., “Resectable Sinonasal Mucosal Melanoma in the Immunotherapy Era: Upfront Surgery vs. Neoadjuvant Therapy,” New Head Neck (2025), 10.1002/hed.28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schenkel J. M., Herbst R. H., Canner D., et al., “Conventional Type I Dendritic Cells Maintain a Reservoir of Proliferative Tumor‐Antigen Specific TCF‐1+ CD8+ T Cells in Tumor‐Draining Lymph Nodes,” Immunity 54, no. 10 (2021): 2338–2353.e6, 10.1016/j.immuni.2021.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang T., Wang C., Wu J., et al., “The Different T‐Cell Receptor Repertoires in Breast Cancer Tumors, Draining Lymph Nodes, and Adjacent Tissues,” Cancer Immunology Research 5, no. 2 (2017): 148–156, 10.1158/2326-6066.CIR-16-0107. [DOI] [PubMed] [Google Scholar]
- 10. Knitz M. W., Bickett T. E., Darragh L. B., et al., “Targeting Resistance to Radiation‐Immunotherapy in Cold HNSCCs by Modulating the Treg‐Dendritic Cell Axis,” Journal for Immunotherapy of Cancer 9, no. 4 (2021): e001955, 10.1136/jitc-2020-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Pul K. M., Fransen M. F., van de Ven R., and de Gruijl T. D., “Immunotherapy Goes Local: The Central Role of Lymph Nodes in Driving Tumor Infiltration and Efficacy,” Frontiers in Immunology 12 (2021): 643291, 10.3389/fimmu.2021.643291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanda Y., Okazaki T., and Katakai T., “Motility Dynamics of T Cells in Tumor‐Draining Lymph Nodes: A Rational Indicator of Antitumor Response and Immune Checkpoint Blockade,” Cancers 13, no. 18 (2021): 4616, 10.3390/cancers13184616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng H., Zhou J., Chen H., et al., “Impact of Lymphadenectomy Extent on Immunotherapy Efficacy in Postresectional Recurred Non‐small Cell Lung Cancer: A Multi‐Institutional Retrospective Cohort Study,” International Journal of Surgery 110, no. 1 (2024): 238–252, 10.1097/JS9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Z. Y., Li Z. Z., Cao L. M., et al., “Seizing the Fate of Lymph Nodes in Immunotherapy: To Preserve or Not?,” Cancer Letters 588 (2024): 216740, 10.1016/j.canlet.2024.216740. [DOI] [PubMed] [Google Scholar]
- 15. Reticker‐Flynn N. E. and Engleman E. G., “Lymph Nodes: At the Intersection of Cancer Treatment and Progression,” Trends in Cell Biology 33, no. 12 (2023): 1021–1034, 10.1016/j.tcb.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang C., Wang Z., Shi L., and Liu W., “Evaluation of Neck Control Strategies for Oral Squamous Cell Carcinoma of Stage I: Neck Dissection or Potential Immunotherapy,” J Dent Sci 19, no. 1 (2024): 640–644, 10.1016/j.jds.2023.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connolly K. A., Kuchroo M., Venkat A., et al., “A Reservoir of Stem‐Like CD8+ T Cells in the Tumor‐Draining Lymph Node Preserves the Ongoing Antitumor Immune Response,” Science Immunology 6, no. 64 (2021): eabg7836, 10.1126/sciimmunol.abg7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Q., Wu X., Wang Z., et al., “The Primordial Differentiation of Tumor‐Specific Memory CD8+ T Cells as Bona Fide Responders to PD‐1/PD‐L1 Blockade in Draining Lymph Nodes,” Cell 185, no. 22 (2022): 4049–4066.e25, 10.1016/j.cell.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 19. Rahim M. K., Okholm T. L. H., Jones K. B., et al., “Dynamic CD8+ T Cell Responses to Cancer Immunotherapy in Human Regional Lymph Nodes Are Disrupted in Metastatic Lymph Nodes,” Cell 186, no. 6 (2023): 1127–1143, 10.1016/j.cell.2023.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janz T. A., Graboyes E. M., Nguyen S. A., et al., “A Comparison of the NCDB and SEER Database for Research Involving Head and Neck Cancer,” Otolaryngol–Head Neck Surg off J Am Acad Otolaryngol‐Head Neck Surg 160, no. 2 (2019): 284–294, 10.1177/0194599818792205. [DOI] [PubMed] [Google Scholar]
- 21. Divi V., Harris J., Harari P. M., et al., “Establishing Quality Indicators for Neck Dissection: Correlating the Number of Lymph Nodes With Oncologic Outcomes (NRG Oncology RTOG 9501 and RTOG 0234),” Cancer 122, no. 22 (2016): 3464–3471, 10.1002/cncr.30204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Divi V., Chen M. M., Nussenbaum B., et al., “Lymph Node Count From Neck Dissection Predicts Mortality in Head and Neck Cancer,” Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 34, no. 32 (2016): 3892–3897, 10.1200/JCO.2016.67.3863. [DOI] [PubMed] [Google Scholar]
- 23. Patel A. M., Haleem A., Revercomb L., et al., “Surgical Resection and Overall Survival in cT4b Sinonasal Non‐squamous Cell Carcinoma,” Laryngoscope Investig Otolaryngol 9, no. 5 (2024): e70025, 10.1002/lio2.70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haimowitz S., Cohen D. A., Dhanda A., et al., “Mucosal Melanoma of the Oral Cavity: What is the Role of Elective Neck Dissection?,” Laryngoscope 133, no. 2 (2023): 317–326, 10.1002/lary.30152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel A. M., Haleem A., Revercomb L., et al., “Primary Site Surgical Resection in cM1 Oral Cavity Squamous Cell Carcinoma,” Laryngoscope Investig Otolaryngol 9, no. 5 (2024): e70000, 10.1002/lio2.70000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leach D. R., Krummel M. F., and Allison J. P., “Enhancement of Antitumor Immunity by CTLA‐4 Blockade,” Science 271, no. 5256 (1996): 1734–1736, 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y. and Zhang Z., “The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor‐Infiltrating Immune Cells and Their Therapeutic Implications,” Cellular & Molecular Immunology 17, no. 8 (2020): 807–821, 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gentilini O. D., Botteri E., Sangalli C., et al., “Sentinel Lymph Node Biopsy vs no Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes,” JAMA Oncology 9, no. 11 (2023): 1557–1564, 10.1001/jamaoncol.2023.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Cruz A. K., Vaish R., Kapre N., et al., “Elective Versus Therapeutic Neck Dissection in Node‐Negative Oral Cancer,” New England Journal of Medicine 373, no. 6 (2015): 521–529, 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 30. Plavc G., Jesenko T., Oražem M., and Strojan P., “Challenges in Combining Immunotherapy With Radiotherapy in Recurrent/Metastatic Head and Neck Cancer,” Cancers 12, no. 11 (2020): 3197, 10.3390/cancers12113197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y., Deng W., Li N., et al., “Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions,” Frontiers in Pharmacology 9 (2018): 185, 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Telarovic I., Yong C. S. M., Kurz L., et al., “Delayed Tumor‐Draining Lymph Node Irradiation Preserves the Efficacy of Combined Radiotherapy and Immune Checkpoint Blockade in Models of Metastatic Disease,” Nature Communications 15, no. 1 (2024): 5500, 10.1038/s41467-024-49873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marciscano A. E., Nirschl T. R., Francica B. J., et al., “Does Prophylactic Nodal Irradiation Inhibit Potential Synergy Between Radiation Therapy and Immunotherapy?,” International Journal of Radiation Oncology, Biology, Physics 96, no. 2 (2016): S88, 10.1016/j.ijrobp.2016.06.222. [DOI] [Google Scholar]
- 34. Marciscano A. E., Ghasemzadeh A., Nirschl T. R., et al., “Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy,” Clinical Cancer Research 24, no. 20 (2018): 5058–5071, 10.1158/1078-0432.CCR-17-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Darragh L. B., Gadwa J., Pham T. T., et al., “Elective Nodal Irradiation Mitigates Local and Systemic Immunity Generated by Combination Radiation and Immunotherapy in Head and Neck Tumors,” Nature Communications 13, no. 1 (2022): 7015, 10.1038/s41467-022-34676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galluzzi L., Aryankalayil M. J., Coleman C. N., and Formenti S. C., “Emerging Evidence for Adapting Radiotherapy to Immunotherapy,” Nature Reviews. Clinical Oncology 20, no. 8 (2023): 543–557, 10.1038/s41571-023-00782-x. [DOI] [PubMed] [Google Scholar]
- 37. Schoenfeld J. D., “Proceed With Caution: Eliminating Elective Nodal Irradiation With Immunotherapy for Head and Neck Cancer,” Int J Radiat Oncol 117, no. 2 (2023): 355–356, 10.1016/j.ijrobp.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 38. Galluzzi L., Humeau J., Buqué A., Zitvogel L., and Kroemer G., “Immunostimulation With Chemotherapy in the Era of Immune Checkpoint Inhibitors,” Nature Reviews. Clinical Oncology 17, no. 12 (2020): 725–741, 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 39. Binnewies M., Mujal A. M., Pollack J. L., et al., “Unleashing Type‐2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity,” Cell 177, no. 3 (2019): 556–571.e16, 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alonso R., Flament H., Lemoine S., et al., “Induction of Anergic or Regulatory Tumor‐Specific CD4+ T Cells in the Tumor‐Draining Lymph Node,” Nature Communications 9, no. 1 (2018): 2113, 10.1038/s41467-018-04524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mortezaee K., “Immune Escape: A Critical Hallmark in Solid Tumors,” Life Sciences 258 (2020): 118110, 10.1016/j.lfs.2020.118110. [DOI] [PubMed] [Google Scholar]
- 42. Van Den Hout M. F. C. M., Koster B. D., Sluijter B. J. R., et al., “Melanoma Sequentially Suppresses Different DC Subsets in the Sentinel Lymph Node, Affecting Disease Spread and Recurrence,” Cancer Immunology Research 5, no. 11 (2017): 969–977, 10.1158/2326-6066.CIR-17-0110. [DOI] [PubMed] [Google Scholar]
- 43. van Krimpen A., Gerretsen V. I. V., Mulder E. E. A. P., et al., “Immune Suppression in the Tumor‐Draining Lymph Node Corresponds With Distant Disease Recurrence in Patients With Melanoma,” Cancer Cell 40 (2022): 798, 10.1016/j.ccell.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 44. Reticker‐Flynn N. E., Zhang W., Belk J. A., et al., “Lymph Node Colonization Induces Tumor‐Immune Tolerance to Promote Distant Metastasis,” Cell 185, no. 11 (2022): 1924–1942, 10.1016/j.cell.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Compérat E., Amin M. B., Cathomas R., et al., “Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments,” Lancet 400, no. 10364 (2022): 1712–1721, 10.1016/S0140-6736(22)01188-6. [DOI] [PubMed] [Google Scholar]
- 46. Patel A. M., Haleem A., Choudhry H. S., Brody R. M., Brant J. A., and Carey R. M., “Elective Neck Dissection in cT1‐4 N0M0 Head and Neck Basaloid Carcinoma,” Otolaryngol–Head Neck Surg off J Am Acad Otolaryngol‐Head Neck Surg 171, no. 2 (2024): 457–470, 10.1002/ohn.757. [DOI] [PubMed] [Google Scholar]
- 47. Enquist I. B., Good Z., Jubb A. M., et al., “Lymph Node‐Independent Liver Metastasis in a Model of Metastatic Colorectal Cancer,” Nature Communications 5, no. 1 (2014): 3530, 10.1038/ncomms4530. [DOI] [PubMed] [Google Scholar]
- 48. Naxerova K., Reiter J. G., Brachtel E., et al., “Origins of Lymphatic and Distant Metastases in Human Colorectal Cancer,” Science 357, no. 6346 (2017): 55–60, 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu J., Deng Y., Liu T., et al., “Lymph Node Metastasis Prediction of Papillary Thyroid Carcinoma Based on Transfer Learning Radiomics,” Nature Communications 11, no. 1 (2020): 4807, 10.1038/s41467-020-18497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu Q., Wang S., Zhang S., et al., “Development of a Deep Learning Model to Identify Lymph Node Metastasis on Magnetic Resonance Imaging in Patients With Cervical Cancer,” JAMA Network Open 3, no. 7 (2020): e2011625, 10.1001/jamanetworkopen.2020.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu S., Hong G., Xu A., et al., “Artificial Intelligence‐Based Model for Lymph Node Metastases Detection on Whole Slide Images in Bladder Cancer: A Retrospective, Multicentre, Diagnostic Study,” Lancet Oncology 24, no. 4 (2023): 360–370, 10.1016/S1470-2045(23)00061-X. [DOI] [PubMed] [Google Scholar]
- 52. Hoshino A., Costa‐Silva B., Shen T. L., et al., “Tumour Exosome Integrins Determine Organotropic Metastasis,” Nature 527, no. 7578 (2015): 329–335, 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saddawi‐Konefka R., O'Farrell A., Faraji F., et al., “Lymphatic‐Preserving Treatment Sequencing With Immune Checkpoint Inhibition Unleashes cDC1‐Dependent Antitumor Immunity in HNSCC,” Nature Communications 13, no. 1 (2022): 4298, 10.1038/s41467-022-31941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leidner R., Crittenden M., Young K., et al., “Neoadjuvant Immunoradiotherapy Results in High Rate of Complete Pathological Response and Clinical to Pathological Downstaging in Locally Advanced Head and Neck Squamous Cell Carcinoma,” Journal for Immunotherapy of Cancer 9, no. 5 (2021): e002485, 10.1136/jitc-2021-002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Buchwald Z. S., Nasti T. H., Lee J., et al., “Tumor‐Draining Lymph Node Is Important for a Robust Abscopal Effect Stimulated by Radiotherapy,” Journal for Immunotherapy of Cancer 8, no. 2 (2020): e000867, 10.1136/jitc-2020-000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prokhnevska N., Cardenas M. A., Valanparambil R. M., et al., “CD8+ T Cell Activation in Cancer Comprises an Initial Activation Phase in Lymph Nodes Followed by Effector Differentiation Within the Tumor,” Immunity 56, no. 1 (2023): 107–124.e5, 10.1016/j.immuni.2022.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cramer J. D., Burtness B., Le Q. T., and Ferris R. L., “The Changing Therapeutic Landscape of Head and Neck Cancer,” Nature Reviews. Clinical Oncology 16, no. 11 (2019): 669–683, 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 58. De Meerleer G., Berghen C., Briganti A., et al., “Elective Nodal Radiotherapy in Prostate Cancer,” Lancet Oncology 22, no. 8 (2021): e348–e357, 10.1016/S1470-2045(21)00242-4. [DOI] [PubMed] [Google Scholar]
- 59. Zhai W. Y., Zhao Z. R., Chen S., et al., “Response of Primary Tumor and Lymph Node in Non‐small Cell Lung Cancer After Neoadjuvant Immunotherapy: A Pooled Analysis,” Journal for Immunotherapy of Cancer 10, no. 9 (2022): e005160, 10.1136/jitc-2022-005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barry E., Schmidt K. L., Topf M. C., and Tassone P., “Addressing the Neck: An NCDB Study of Clinically Node‐Negative Supraglottic Squamous Cell Carcinoma,” Otolaryngology–Head and Neck Surgery 171, no. 5 (2024): 1451–1461, 10.1002/ohn.932. [DOI] [PubMed] [Google Scholar]
- 61. Linton O. R., Moore M. G., Brigance J. S., Gordon C. A., Summerlin D. J., and McDonald M. W., “Prognostic Significance of Basaloid Squamous Cell Carcinoma in Head and Neck Cancer,” JAMA Otolaryngology. Head & Neck Surgery 139, no. 12 (2013): 1306–1311, 10.1001/jamaoto.2013.5308. [DOI] [PubMed] [Google Scholar]
- 62. Joseph A. W. and D'Souza G., “Epidemiology of Human Papillomavirus‐Related Head and Neck Cancer,” Otolaryngology Clinics of North America 45, no. 4 (2012): 739–764, 10.1016/j.otc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 63. O'Rorke M. A., Ellison M. V., Murray L. J., Moran M., James J., and Anderson L. A., “Human Papillomavirus Related Head and Neck Cancer Survival: A Systematic Review and Meta‐Analysis,” Oral Oncology 48, no. 12 (2012): 1191–1201, 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 64. Ang K. K., Harris J., Wheeler R., et al., “Human Papillomavirus and Survival of Patients With Oropharyngeal Cancer,” New England Journal of Medicine 363, no. 1 (2010): 24–35, 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Petrelli F., Sarti E., and Barni S., “Predictive Value of Human Papillomavirus in Oropharyngeal Carcinoma Treated With Radiotherapy: An Updated Systematic Review and Meta‐Analysis of 30 Trials,” Head & Neck 36, no. 5 (2014): 750–759, 10.1002/hed.23351. [DOI] [PubMed] [Google Scholar]
- 66. Li H., Torabi S. J., Yarbrough W. G., Mehra S., Osborn H. A., and Judson B., “Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites With Overall Survival,” JAMA Otolaryngology. Head & Neck Surgery 144, no. 6 (2018): 519–525, 10.1001/jamaoto.2018.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]


