Abstract
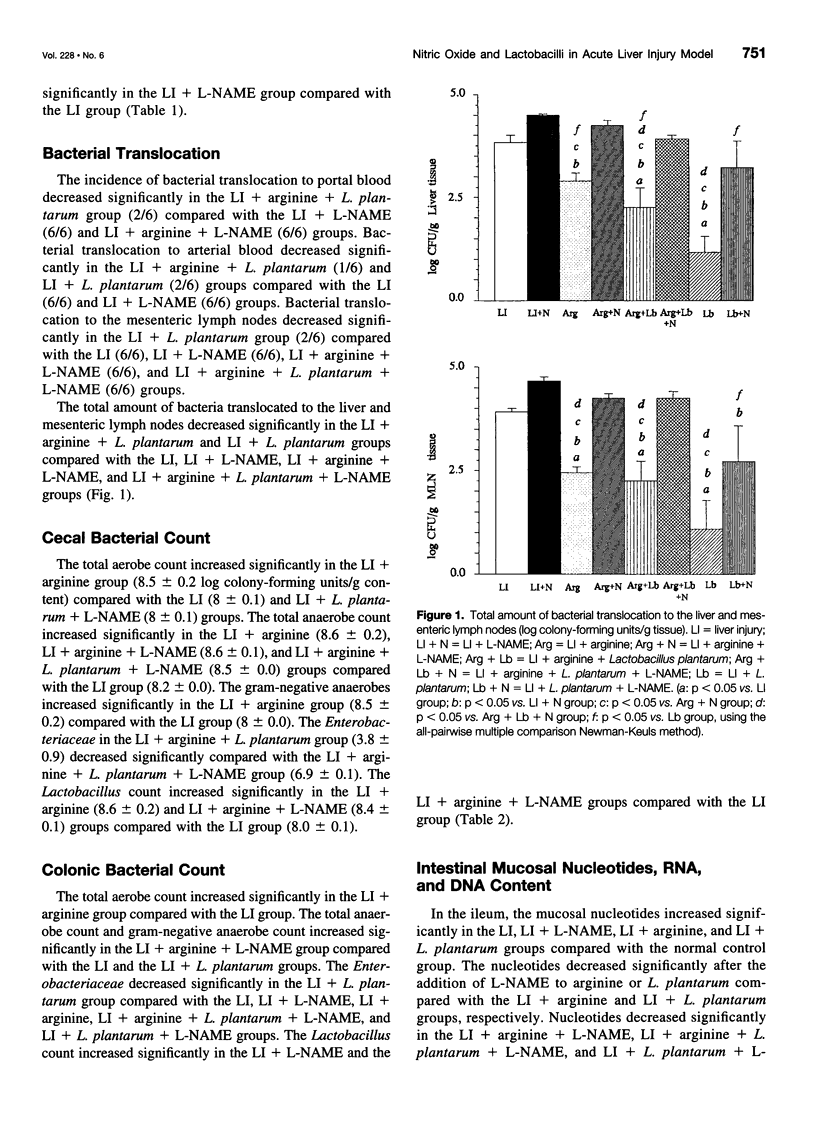
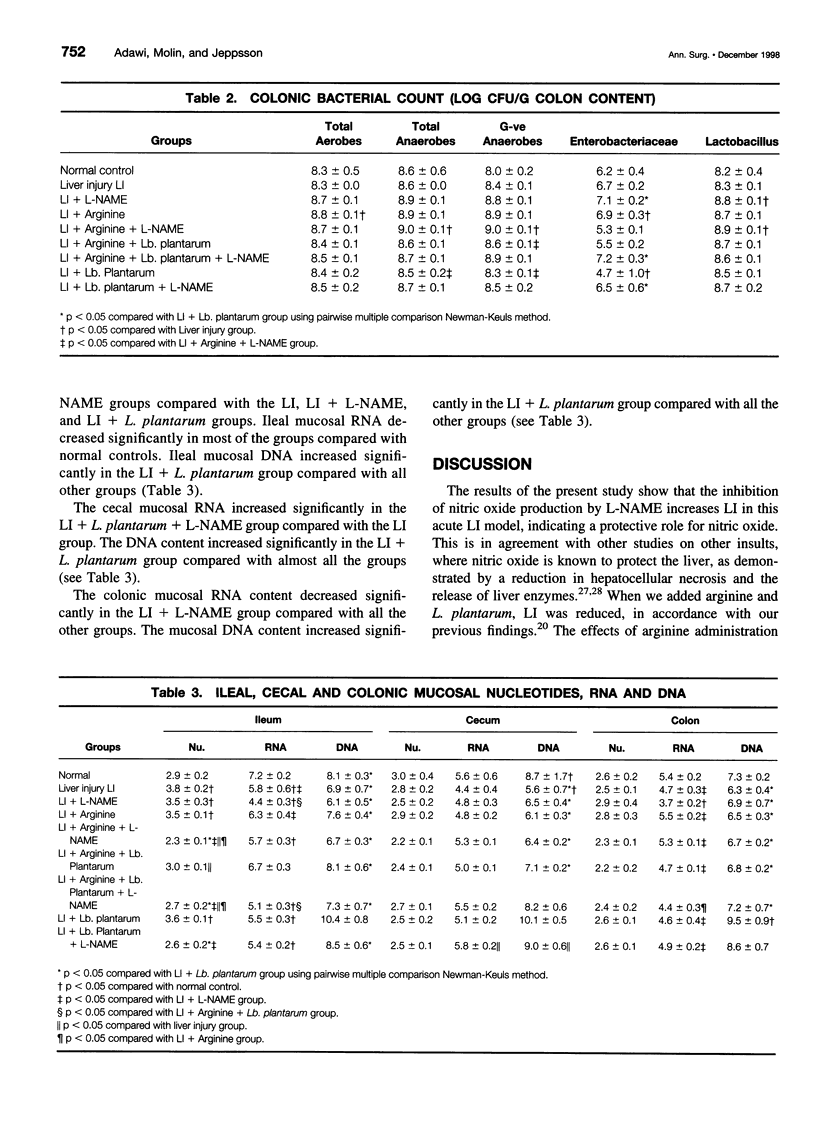
OBJECTIVE: To study the effect of inhibiting nitric oxide production and the effects of arginine and lactobacilli administration in an acute liver injury (LI) model. SUMMARY BACKGROUND DATA: Infectious complications caused by enteric bacteria are common in patients with liver diseases and those who have undergone liver surgery. Increased bacterial translocation has been proposed as one underlying mechanism. Lactobacilli constitute an integral part of the normal gastrointestinal microecology; they are involved in host metabolism and have many beneficial properties. Arginine has numerous roles in cellular metabolism and may be metabolized by lactobacilli in some cases. We have previously shown that rectal administration of Lactobacillus plantarum DSM 9843 (strain 299v), with and without arginine, in an acute LI model significantly reduces the extent of the LI and reduces bacterial translocation. To clarify the pathogenetic mechanisms, we studied the role of nitric oxide in the effects of L. plantarum and arginine in acute LI, as determined by bacterial translocation, ileal, cecal, and colonic nucleotides, RNA, and DNA. METHODS: Male Sprague-Dawley rats were used. L. plantarum, 2% arginine, and/or N-nitro-L-arginine methyl ester (L-NAME), as appropriate, were administered rectally once daily for 8 days. Acute LI was induced on the eighth day by intraperitoneal injection of D-galactosamine (1.1 g/kg body weight), and samples were collected after 24 hours. Bacterial translocation was evaluated by culture of portal and arterial blood, mesenteric lymph nodes, and liver tissue. Liver enzymes and bilirubin were assayed in the serum. The bacterial load in the cecum and colon was determined. Ileal, cecal, and colonic mucosal nucleotides, RNA, and DNA were evaluated. RESULTS: The levels of liver enzymes and bilirubin were lower in liver-injured rats supplemented with arginine and Lactobacillus, and this effect was abolished by the addition of L-NAME. Inhibition of nitric oxide production (by L-NAME) increased bacterial translocation in many groups. L-NAME administration increased the cecal and colonic bacterial count and decreased the levels of mucosal nucleotides, RNA, and DNA. CONCLUSIONS: Inhibition of nitric oxide production modulated the effects of arginine and L. plantarum in this acute LI model. L-NAME potentiated the LI, as indicated by elevation of liver enzymes and bilirubin, and it also increased bacterial translocation and the cecal and colonic bacterial count. Increased bacterial translocation could be one of the mechanisms by which LI is potentiated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Adawi D., Kasravi F. B., Molin G., Jeppsson B. Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology. 1997 Mar;25(3):642–647. doi: 10.1002/hep.510250325. [DOI] [PubMed] [Google Scholar]
- Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 1986 Mar-Apr;10(2):227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Barbul A., Sisto D. A., Wasserkrug H. L., Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981 Aug;90(2):244–251. [PubMed] [Google Scholar]
- Billiar T. R., Langrehr J. M., Curran R. D., Ochoa J. B., Stadler J., Harbrecht B. G., Hoffman R. A., Simmons R. L. Two unique aspects of inducible .N = O synthase in liver cells and accessory cells: hepatic damage is minimized by hepatocyte .N = O production and immunoregulation is mediated by macrophage .N = O production. Res Immunol. 1991 Sep;142(7):584–599. doi: 10.1016/0923-2494(91)90108-u. [DOI] [PubMed] [Google Scholar]
- Bismuth H., Samuel D., Gugenheim J., Castaing D., Bernuau J., Rueff B., Benhamou J. P. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med. 1987 Sep;107(3):337–341. doi: 10.7326/0003-4819-107-2-337. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Hutcheson I. R., Deakin A. M., Whittle B. J., Moncada S. Protective effect of S-nitroso-N-acetyl-penicillamine in endotoxin-induced acute intestinal damage in the rat. Eur J Pharmacol. 1990 Dec 4;191(3):485–488. doi: 10.1016/0014-2999(90)94185-z. [DOI] [PubMed] [Google Scholar]
- Daly J. M., Reynolds J., Thom A., Kinsley L., Dietrick-Gallagher M., Shou J., Ruggieri B. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988 Oct;208(4):512–523. doi: 10.1097/00000658-198810000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. Probiotics in human medicine. Gut. 1991 Apr;32(4):439–442. doi: 10.1136/gut.32.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad R. A., Wright N. A. Changes in intestinal cell proliferation, absorptive capacity and structure in young, adult and old rats. J Anat. 1990 Dec;173:109–118. [PMC free article] [PubMed] [Google Scholar]
- Green S. J. Nitric oxide in mucosal immunity. Nat Med. 1995 Jun;1(6):515–517. doi: 10.1038/nm0695-515. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995 Dec;59(4):533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. L., Molin G., Jeppsson B., Nobaek S., Ahrné S., Bengmark S. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl Environ Microbiol. 1993 Jan;59(1):15–20. doi: 10.1128/aem.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Carson J., Vazquez-Torres A., van der Heyde H. C., Warner T., Wagner R. D., Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995 Jun;1(6):552–557. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- Kanwar S., Wallace J. L., Befus D., Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994 Feb;266(2 Pt 1):G222–G229. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- Kelly E., Morris S. M., Jr, Billiar T. R. Nitric oxide, sepsis, and arginine metabolism. JPEN J Parenter Enteral Nutr. 1995 May-Jun;19(3):234–238. doi: 10.1177/0148607195019003234. [DOI] [PubMed] [Google Scholar]
- Kuo P. C., Slivka A. Nitric oxide decreases oxidant-mediated hepatocyte injury. J Surg Res. 1994 Jun;56(6):594–600. doi: 10.1006/jsre.1994.1094. [DOI] [PubMed] [Google Scholar]
- Manca de Nadra M. C., Pesce de Ruiz Holgado A. A., Oliver G. Arginine dihydrolase pathway in Lactobacillus buchneri: a review. Biochimie. 1988 Mar;70(3):367–374. doi: 10.1016/0300-9084(88)90209-x. [DOI] [PubMed] [Google Scholar]
- Mao Y., Nobaek S., Kasravi B., Adawi D., Stenram U., Molin G., Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996 Aug;111(2):334–344. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Perdigon G., de Macias M. E., Alvarez S., Oliver G., de Ruiz Holgado A. A. Effect of perorally administered lactobacilli on macrophage activation in mice. Infect Immun. 1986 Aug;53(2):404–410. doi: 10.1128/iai.53.2.404-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakela J., Lange S. M., Ludwig J., Baldus W. P. Fulminant hepatitis: Mayo Clinic experience with 34 cases. Mayo Clin Proc. 1985 May;60(5):289–292. doi: 10.1016/s0025-6196(12)60534-5. [DOI] [PubMed] [Google Scholar]
- SCOTT J. F., FRACCASTORO A. P., TAFT E. B. Studies in histochemistry. I. Determination of nucleic acids in microgram amounts of tissue. J Histochem Cytochem. 1956 Jan;4(1):1–10. doi: 10.1177/4.1.1. [DOI] [PubMed] [Google Scholar]
- Sato K., Saito H., Tomioka H. Enhancement of host resistance against Listeria infection by Lactobacillus casei: activation of liver macrophages and peritoneal macrophages by Lactobacillus casei. Microbiol Immunol. 1988;32(7):689–698. doi: 10.1111/j.1348-0421.1988.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Silva M., Jacobus N. V., Deneke C., Gorbach S. L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987 Aug;31(8):1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober S., Slavin S., Fuks Z., Kaplan H. S., Gottlieb M., Bieber C., Hoppe R. T., Grumet F. C. Transplantation tolerance after total lymphoid irradiation. Transplant Proc. 1979 Mar;11(1):1032–1038. [PubMed] [Google Scholar]
- Unno N., Wang H., Menconi M. J., Tytgat S. H., Larkin V., Smith M., Morin M. J., Chavez A., Hodin R. A., Fink M. P. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997 Oct;113(4):1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- Vos T. A., Gouw A. S., Klok P. A., Havinga R., van Goor H., Huitema S., Roelofsen H., Kuipers F., Jansen P. L., Moshage H. Differential effects of nitric oxide synthase inhibitors on endotoxin-induced liver damage in rats. Gastroenterology. 1997 Oct;113(4):1323–1333. doi: 10.1053/gast.1997.v113.pm9322528. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990 Mar;99(3):607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


