Abstract
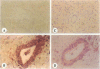
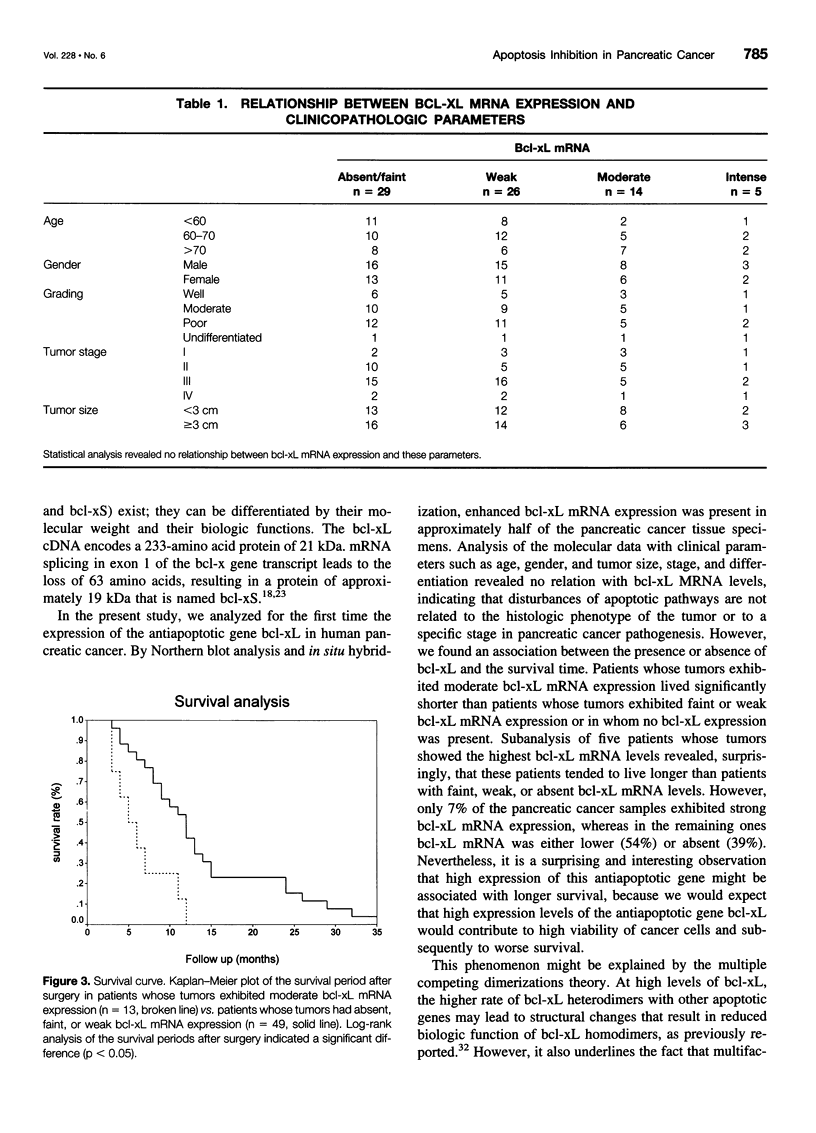
OBJECTIVE: To analyze the expression of the antiapoptotic gene bcl-xL in human pancreatic cancer and to correlate the results with clinical patient parameters. SUMMARY BACKGROUND DATA: Bcl-xL belongs to the bcl-2-related gene family and acts as a broad antiapoptotic factor to extend both normal and tumor cell survival. Recent findings indicate that tumor cell death induced by chemotherapy and radiotherapy is mediated by the activation of apoptosis. The fact that pancreatic cancer has an extremely malignant potential and that it is resistant to most anticancer treatment modalities suggests that mechanisms are activated that increase the viability of pancreatic cancer cells. METHODS: Seventy-four pancreatic cancer tissue samples were obtained from 32 female and 42 male patients undergoing surgery for exocrine pancreatic cancer. Normal human pancreatic tissue samples were available from 11 organ donors and 4 patients without pancreatic disease. The levels of bcl-xL mRNA expression were analyzed by Northern blot analysis. The exact site of bcl-xL mRNA transcription was determined by nonradioactive in situ hybridization. In addition, immunohistochemistry using specific polyclonal antibodies was used to localize the protein. RESULTS: Northern blot analysis indicated that, in comparison with the normal pancreas, bcl-xL mRNA was markedly overexpressed in 54% of the pancreatic cancer samples. Densitometric analysis revealed that pancreatic adenocarcinomas exhibited a mean 3.4-fold increase (p < 0.01) in bcl-xL mRNA levels in comparison with normal controls. With in situ hybridization, bcl-xL mRNA was found to be highly expressed in the cancer cells of tumor samples that exhibited increased mRNA expression by Northern blot analysis. Immunohistochemical analysis revealed bcl-x immunostaining in 88% of the cancer samples. Correlation of the molecular data with clinical patient parameters revealed that patients whose tumors exhibited no, faint, or weak bcl-xL expression lived significantly longer after tumor resection (median 12 months) than patients whose tumors exhibited moderate bcl-xL mRNA expression (median 5 months) (p < 0.05). However, 5 patients whose tumors exhibited intense bcl-xL mRNA expression tended to live longer (median 14 months). CONCLUSION: Enhanced expression of the antiapoptotic gene bcl-xL in pancreatic cancer and its association with shorter patient survival suggests that this factor may enhance the viability of pancreatic cancer cells in vivo. Inhibition of apoptotic pathways might be one of the reasons why pancreatic cancer shows only limited sensitivity to anticancer treatment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrén-Sandberg A., Bäckman P. L., Andersson R. Results of adjuvant therapy in resected pancreatic cancer. Int J Pancreatol. 1997 Feb;21(1):31–38. doi: 10.1007/BF02785917. [DOI] [PubMed] [Google Scholar]
- Beger H. G., Büchler M. W., Friess H. Chirurgische Ergebnisse und Indikation zu adjuvanten Massnahmen beim Pankreascarcinom. Chirurg. 1994 Apr;65(4):246–252. [PubMed] [Google Scholar]
- Berthélemy P., Bouisson M., Escourrou J., Vaysse N., Rumeau J. L., Pradayrol L. Identification of K-ras mutations in pancreatic juice in the early diagnosis of pancreatic cancer. Ann Intern Med. 1995 Aug 1;123(3):188–191. doi: 10.7326/0003-4819-123-3-199508010-00005. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Büchler M., Friess H., Schultheiss K. H., Gebhardt C., Kübel R., Muhrer K. H., Winkelmann M., Wagener T., Klapdor R., Kaul M. A randomized controlled trial of adjuvant immunotherapy (murine monoclonal antibody 494/32) in resectable pancreatic cancer. Cancer. 1991 Oct 1;68(7):1507–1512. doi: 10.1002/1097-0142(19911001)68:7<1507::aid-cncr2820680707>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Cantero D., Friess H., Deflorin J., Zimmermann A., Bründler M. A., Riesle E., Korc M., Büchler M. W. Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer. 1997;75(3):388–395. doi: 10.1038/bjc.1997.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng E. H., Levine B., Boise L. H., Thompson C. B., Hardwick J. M. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996 Feb 8;379(6565):554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- Cherbonnel-Lasserre C., Gauny S., Kronenberg A. Suppression of apoptosis by Bcl-2 or Bcl-xL promotes susceptibility to mutagenesis. Oncogene. 1996 Oct 3;13(7):1489–1497. [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Datta R., Manome Y., Taneja N., Boise L. H., Weichselbaum R., Thompson C. B., Slapak C. A., Kufe D. Overexpression of Bcl-XL by cytotoxic drug exposure confers resistance to ionizing radiation-induced internucleosomal DNA fragmentation. Cell Growth Differ. 1995 Apr;6(4):363–370. [PubMed] [Google Scholar]
- Dole M. G., Jasty R., Cooper M. J., Thompson C. B., Nuñez G., Castle V. P. Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res. 1995 Jun 15;55(12):2576–2582. [PubMed] [Google Scholar]
- Foreman K. E., Wrone-Smith T., Boise L. H., Thompson C. B., Polverini P. J., Simonian P. L., Nunez G., Nickoloff B. J. Kaposi's sarcoma tumor cells preferentially express Bcl-xL. Am J Pathol. 1996 Sep;149(3):795–803. [PMC free article] [PubMed] [Google Scholar]
- Friess H., Berberat P., Schilling M., Kunz J., Korc M., Büchler M. W. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med (Berl) 1996 Jan;74(1):35–42. doi: 10.1007/BF00202070. [DOI] [PubMed] [Google Scholar]
- Friess H., Büchler M., Krüger M., Beger H. G. Treatment of duct carcinoma of the pancreas with the LH-RH analogue buserelin. Pancreas. 1992;7(5):516–521. doi: 10.1097/00006676-199209000-00002. [DOI] [PubMed] [Google Scholar]
- Friess H., Cantero D., Graber H., Tang W. H., Guo X., Kashiwagi M., Zimmermann A., Gold L., Korc M., Büchler M. W. Enhanced urokinase plasminogen activation in chronic pancreatitis suggests a role in its pathogenesis. Gastroenterology. 1997 Sep;113(3):904–913. doi: 10.1016/s0016-5085(97)70186-0. [DOI] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Berger H. G., Kobrin M. S., Baldwin R. L., Korc M. Enhanced expression of the type II transforming growth factor beta receptor in human pancreatic cancer cells without alteration of type III receptor expression. Cancer Res. 1993 Jun 15;53(12):2704–2707. [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Ebert M., Beger H. G., Gold L. I., Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993 Dec;105(6):1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- Gauthier E. R., Piché L., Lemieux G., Lemieux R. Role of bcl-X(L) in the control of apoptosis in murine myeloma cells. Cancer Res. 1996 Mar 15;56(6):1451–1456. [PubMed] [Google Scholar]
- Gottschalk A. R., Boise L. H., Thompson C. B., Quintáns J. Identification of immunosuppressant-induced apoptosis in a murine B-cell line and its prevention by bcl-x but not bcl-2. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress T. M., Müller-Pillasch F., Lerch M. M., Friess H., Büchler M., Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995 Aug 9;62(4):407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- Guo X., Friess H., Graber H. U., Kashiwagi M., Zimmermann A., Korc M., Büchler M. W. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996 Nov 1;56(21):4876–4880. [PubMed] [Google Scholar]
- Huang D. C., O'Reilly L. A., Strasser A., Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997 Aug 1;16(15):4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihse I., Anderson H., Andrén-Sandberg Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996 Mar-Apr;20(3):288–294. doi: 10.1007/s002689900046. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shinomura Y., Kanayama S., Higashimoto Y., Miyagawa J. I., Minami T., Kiyohara T., Zushi S., Kitamura S., Isozaki K. Over-expression of bcl-xL gene in human gastric adenomas and carcinomas. Int J Cancer. 1996 Dec 11;68(6):727–730. doi: 10.1002/(SICI)1097-0215(19961211)68:6<727::AID-IJC6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Korsmeyer S. J., Shutter J. R., Veis D. J., Merry D. E., Oltvai Z. N. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993 Dec;4(6):327–332. [PubMed] [Google Scholar]
- Krajewska M., Moss S. F., Krajewski S., Song K., Holt P. R., Reed J. C. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996 May 15;56(10):2422–2427. [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Wang H. G., Irie S., Fong L., Reed J. C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994 Nov 1;54(21):5501–5507. [PubMed] [Google Scholar]
- Lu Z., Friess H., Graber H. U., Guo X., Schilling M., Zimmermann A., Korc M., Büchler M. W. Presence of two signaling TGF-beta receptors in human pancreatic cancer correlates with advanced tumor stage. Dig Dis Sci. 1997 Oct;42(10):2054–2063. doi: 10.1023/a:1018814416903. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Beham A., Sarkiss M., Andersen M. M., Lo P. Importance of the Bcl-2 family in cell death regulation. Experientia. 1996 Oct 31;52(10-11):1008–1017. doi: 10.1007/BF01920110. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- O'Reilly L. A., Huang D. C., Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996 Dec 16;15(24):6979–6990. [PMC free article] [PubMed] [Google Scholar]
- Offit K., Wong G., Filippa D. A., Tao Y., Chaganti R. S. Cytogenetic analysis of 434 consecutively ascertained specimens of non-Hodgkin's lymphoma: clinical correlations. Blood. 1991 Apr 1;77(7):1508–1515. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Palmer K. R., Kerr M., Knowles G., Cull A., Carter D. C., Leonard R. C. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994 Jun;81(6):882–885. doi: 10.1002/bjs.1800810629. [DOI] [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1997. CA Cancer J Clin. 1997 Jan-Feb;47(1):5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Miyashita T., Takayama S., Wang H. G., Sato T., Krajewski S., Aimé-Sempé C., Bodrug S., Kitada S., Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996 Jan;60(1):23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Riesle E., Friess H., Zhao L., Wagner M., Uhl W., Baczako K., Gold L. I., Korc M., Büchler M. W. Increased expression of transforming growth factor beta s after acute oedematous pancreatitis in rats suggests a role in pancreatic repair. Gut. 1997 Jan;40(1):73–79. doi: 10.1136/gut.40.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott A. F., Apel I. J., Nuñez G., Clarke M. F. Bcl-XL protects cancer cells from p53-mediated apoptosis. Oncogene. 1995 Oct 5;11(7):1389–1394. [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995 Jan 27;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Mayer M. G., Arnesen M. A., Aeppli D. P., Oken M. M., Frizzera G. bcl-2 and other genomic alterations in the prognosis of large-cell lymphoma. N Engl J Med. 1989 Apr 20;320(16):1047–1054. doi: 10.1056/NEJM198904203201605. [DOI] [PubMed] [Google Scholar]