Abstract
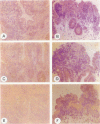
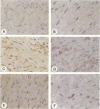
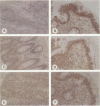
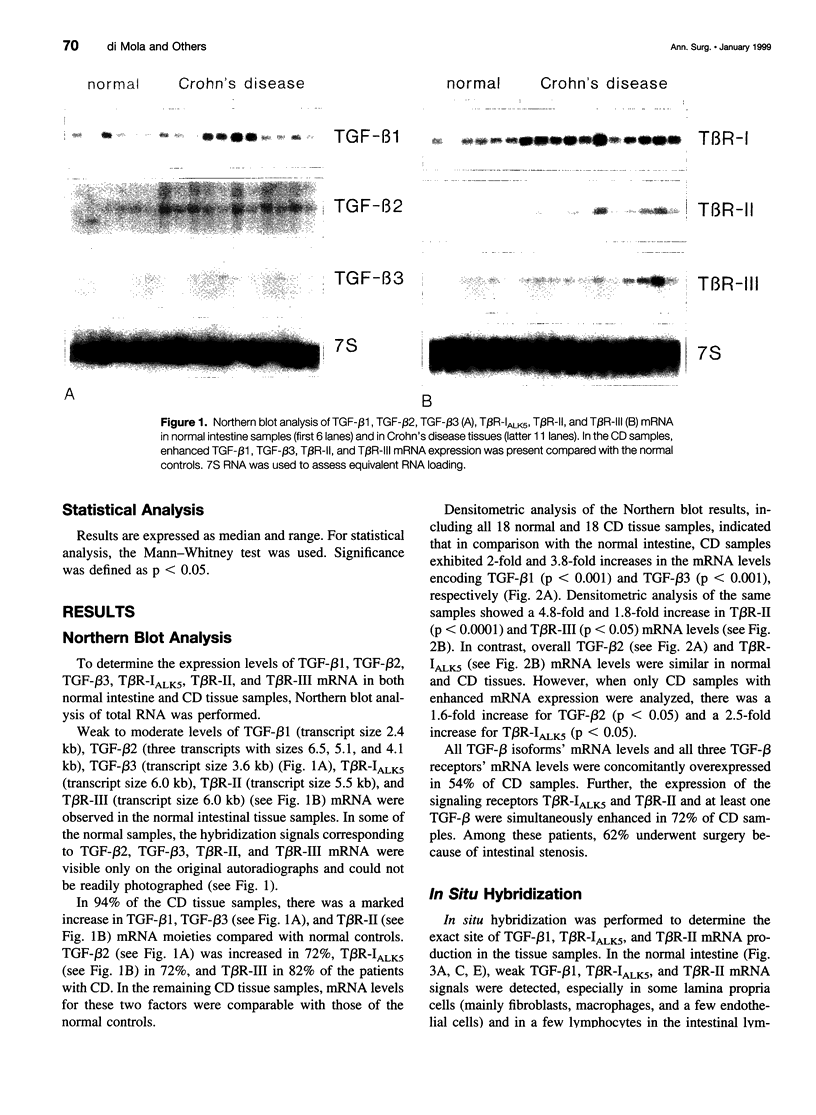
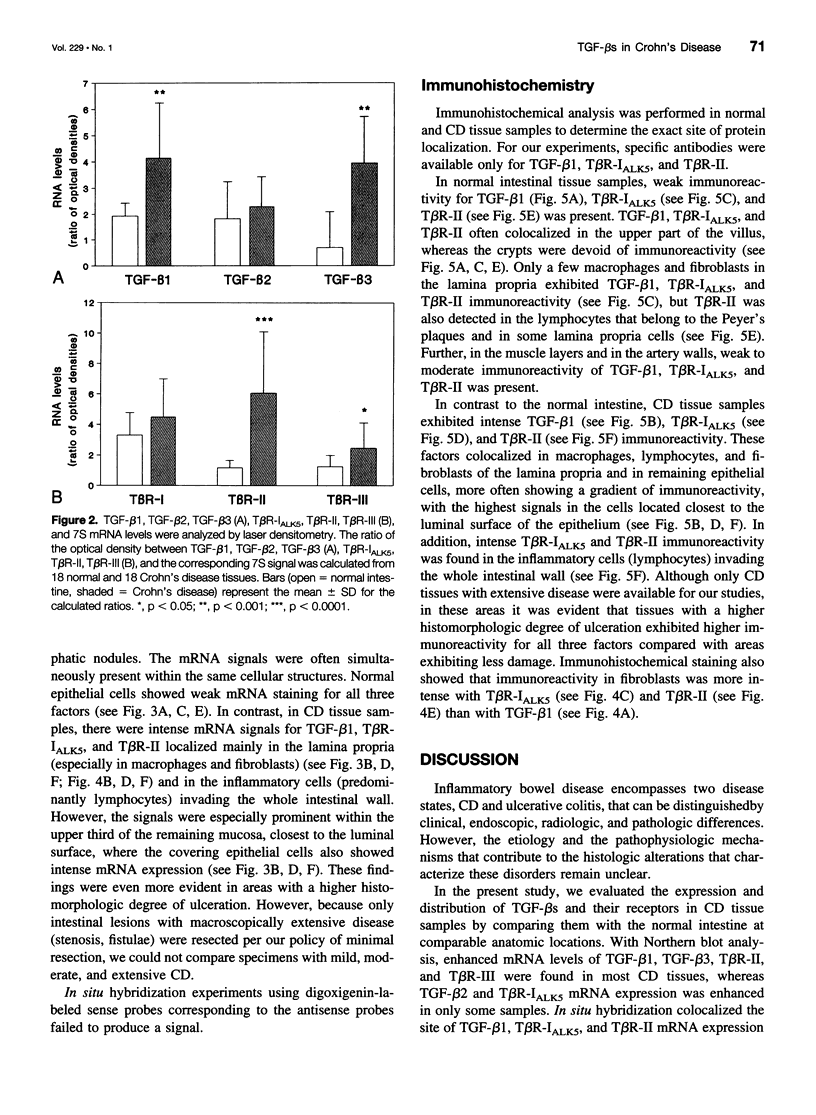
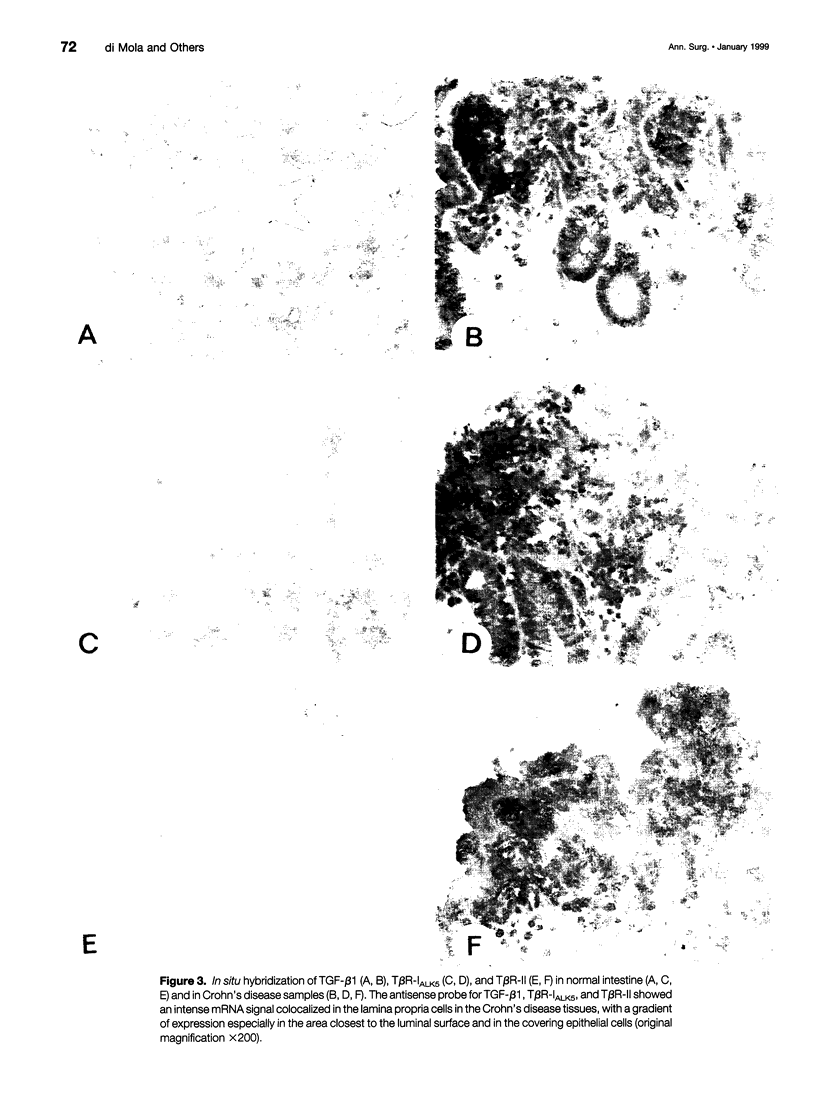
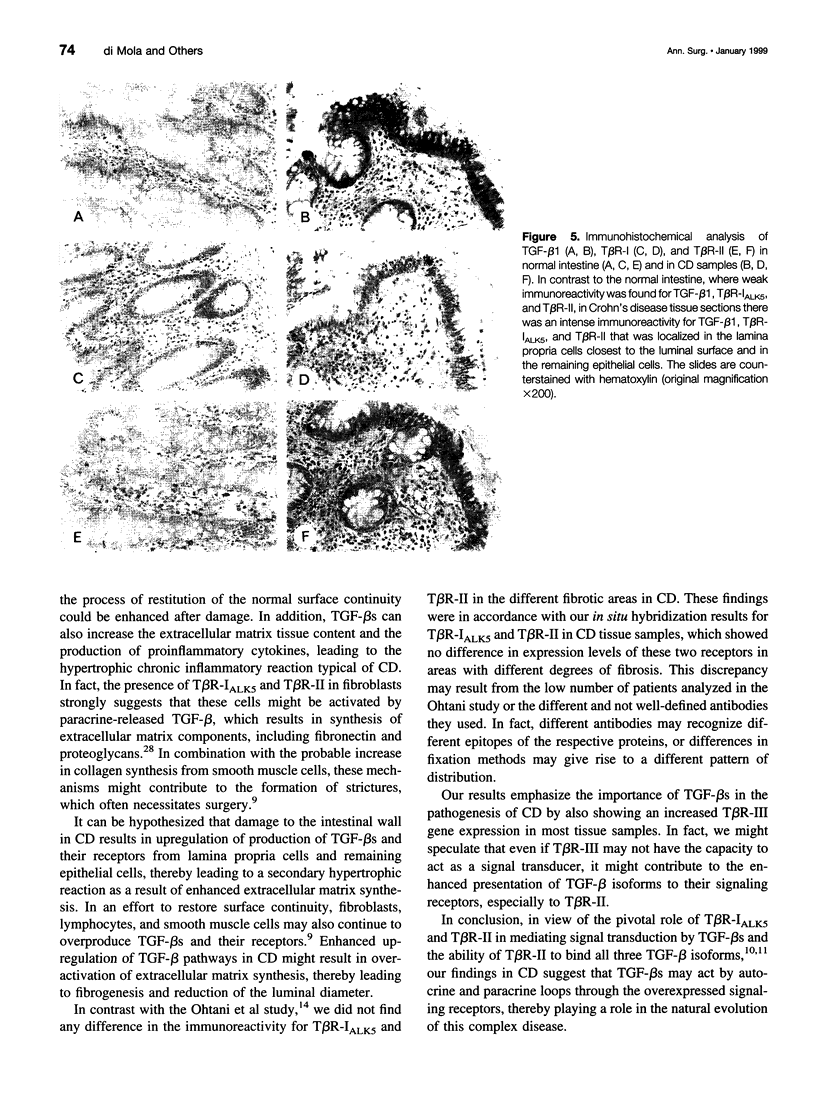
OBJECTIVE: To evaluate mechanisms that contribute to tissue repair and tissue remodeling in Crohn's disease (CD). SUMMARY BACKGROUND DATA: Transforming growth factor-betas (TGF-betas) are involved in different chronic inflammatory disorders. They function by binding to two receptors, type I (TbetaR-I) subtype ALK5 and type II (TbetaR-II), which are concomitantly required for signal transduction. METHODS: Tissues were obtained from 18 patients with CD (10 female patients, 8 male patients, median age 38.7 years [range 16 to 58 years]) undergoing surgery because of CD-related complications. Tissue samples of 18 healthy organ donors (10 female subjects, 8 male subjects, median age 50.3 years [range 15 to 65 years]) served as controls. The expression and localization of TGF-beta1, TGF-beta2, TGF-beta3, TbetaR-IALK5, TbetaR-II, and TbetaR-III were studied by Northern blot analysis, in situ hybridization, and immunohistochemistry. RESULTS: On Northern blot analysis, 94% of the CD samples exhibited enhanced TGF-beta1, TGF-beta3, and TbetaR-II mRNA expression compared with controls. TGF-beta2 was increased in 72%, TbetaR-IALK5 in 72%, and TbetaR-III in 82% of the patients with CD. On in situ hybridization and immunohistochemical analysis, TGF-beta1, TbetaR-IALK5, and TbetaR-II were seen to be colocalized in the lamina propria cells and in the lymphocytes closest to the luminal surface, but also in the remaining epithelial cells, and in fibroblasts of CD tissue samples. CONCLUSIONS: The concomitant overexpression of TGF-betas and their signaling receptors in CD points to a potential role of these regulatory molecules in the pathophysiology of CD. Activation of TGF-beta-mediated pathways might promote the repair of mucosal injury by enhancing the process of reepithelization, but might also contribute to extracellular matrix generation and subsequently to intramural fibrosis and intestinal obstruction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. J., Panja A., Kaplan-Liss E., Mayer L., Raicht R. F. Expression of growth factor receptor-encoded mRNA by colonic epithelial cells is altered in inflammatory bowel disease. Dig Dis Sci. 1995 Mar;40(3):485–494. doi: 10.1007/BF02064355. [DOI] [PubMed] [Google Scholar]
- Babyatsky M. W., Rossiter G., Podolsky D. K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996 Apr;110(4):975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Friess H., Yokoyama M., Lopez M. E., Kobrin M. S., Büchler M. W., Korc M. Attenuated ALK5 receptor expression in human pancreatic cancer: correlation with resistance to growth inhibition. Int J Cancer. 1996 Jul 17;67(2):283–288. doi: 10.1002/(SICI)1097-0215(19960717)67:2<283::AID-IJC21>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Breese E. J., Michie C. A., Nicholls S. W., Murch S. H., Williams C. B., Domizio P., Walker-Smith J. A., MacDonald T. T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994 Jun;106(6):1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Berger H. G., Kobrin M. S., Baldwin R. L., Korc M. Enhanced expression of the type II transforming growth factor beta receptor in human pancreatic cancer cells without alteration of type III receptor expression. Cancer Res. 1993 Jun 15;53(12):2704–2707. [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Ebert M., Beger H. G., Gold L. I., Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993 Dec;105(6):1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- Garcia-González M., Boixeda D., Herrero D., Burgaleta C. Effect of granulocyte-macrophage colony-stimulating factor on leukocyte function in cirrhosis. Gastroenterology. 1993 Aug;105(2):527–531. doi: 10.1016/0016-5085(93)90730-z. [DOI] [PubMed] [Google Scholar]
- Graham M. F., Bryson G. R., Diegelmann R. F. Transforming growth factor beta 1 selectively augments collagen synthesis by human intestinal smooth muscle cells. Gastroenterology. 1990 Aug;99(2):447–453. doi: 10.1016/0016-5085(90)91028-5. [DOI] [PubMed] [Google Scholar]
- Guo X., Friess H., Graber H. U., Kashiwagi M., Zimmermann A., Korc M., Büchler M. W. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996 Nov 1;56(21):4876–4880. [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Flanders K. C., Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996 Feb;14(2):131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- Kurokowa M., Lynch K., Podolsky D. K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Payne H. M., Andres J. L., Massagué J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J Cell Biol. 1994 Feb;124(4):557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McCabe R. P., Secrist H., Botney M., Egan M., Peters M. G. Cytokine mRNA expression in intestine from normal and inflammatory bowel disease patients. Clin Immunol Immunopathol. 1993 Jan;66(1):52–58. doi: 10.1006/clin.1993.1007. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Biancone L., Marasco R., Morrone G., Marasco O., Luzza F., Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997 Apr;112(4):1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- Ohtani H., Kagaya H., Nagura H. Immunohistochemical localization of transforming growth factor-beta receptors I and II in inflammatory bowel disease. J Gastroenterol. 1995 Nov;30 (Suppl 8):76–77. [PubMed] [Google Scholar]
- Reibman J., Meixler S., Lee T. C., Gold L. I., Cronstein B. N., Haines K. A., Kolasinski S. L., Weissmann G. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6805–6809. doi: 10.1073/pnas.88.15.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S. N., Ghosh N. Reversible unfolding of Escherichia coli alkaline phosphatase: active site can be reconstituted by a number of pathways. Arch Biochem Biophys. 1996 Jun 1;330(1):174–180. doi: 10.1006/abbi.1996.0239. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- van Hees P. A., van Elteren P. H., van Lier H. J., van Tongeren J. H. An index of inflammatory activity in patients with Crohn's disease. Gut. 1980 Apr;21(4):279–286. doi: 10.1136/gut.21.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Laethem J. L., Deviere J., Resibois A., Rickaert F., Vertongen P., Ohtani H., Cremer M., Miyazono K., Robberecht P. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology. 1995 Jun;108(6):1873–1881. doi: 10.1016/0016-5085(95)90152-3. [DOI] [PubMed] [Google Scholar]