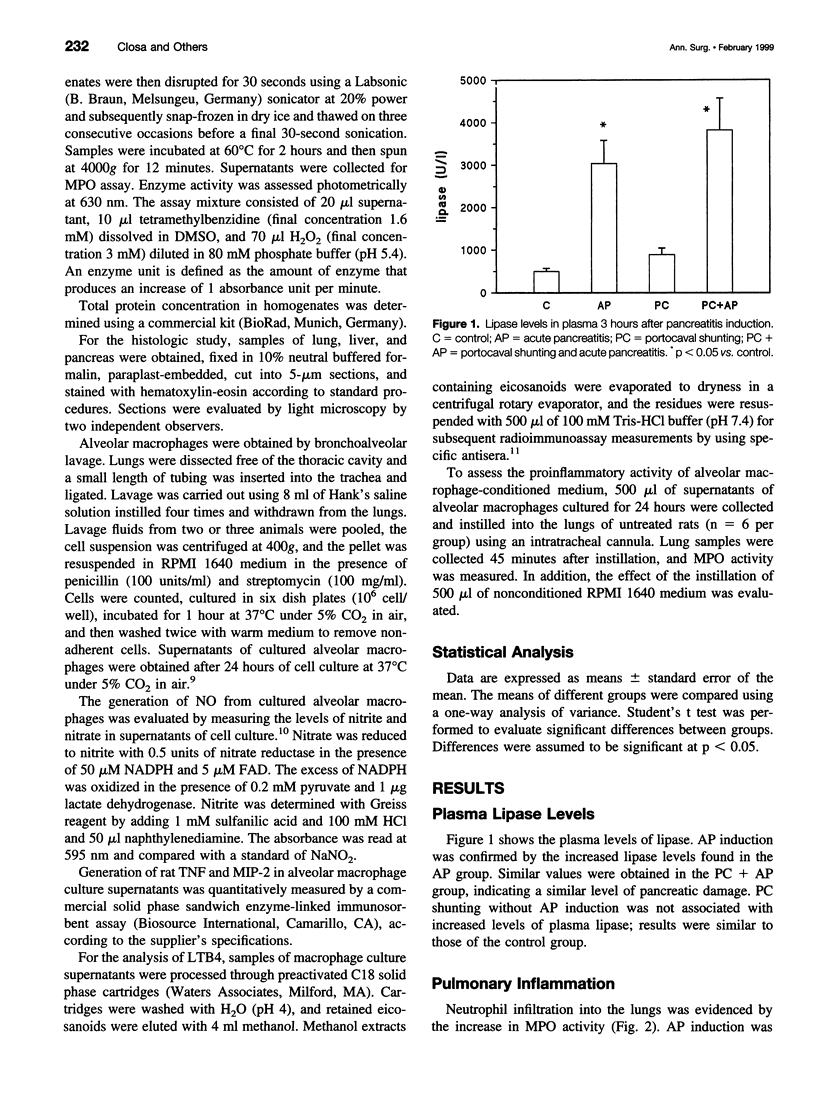
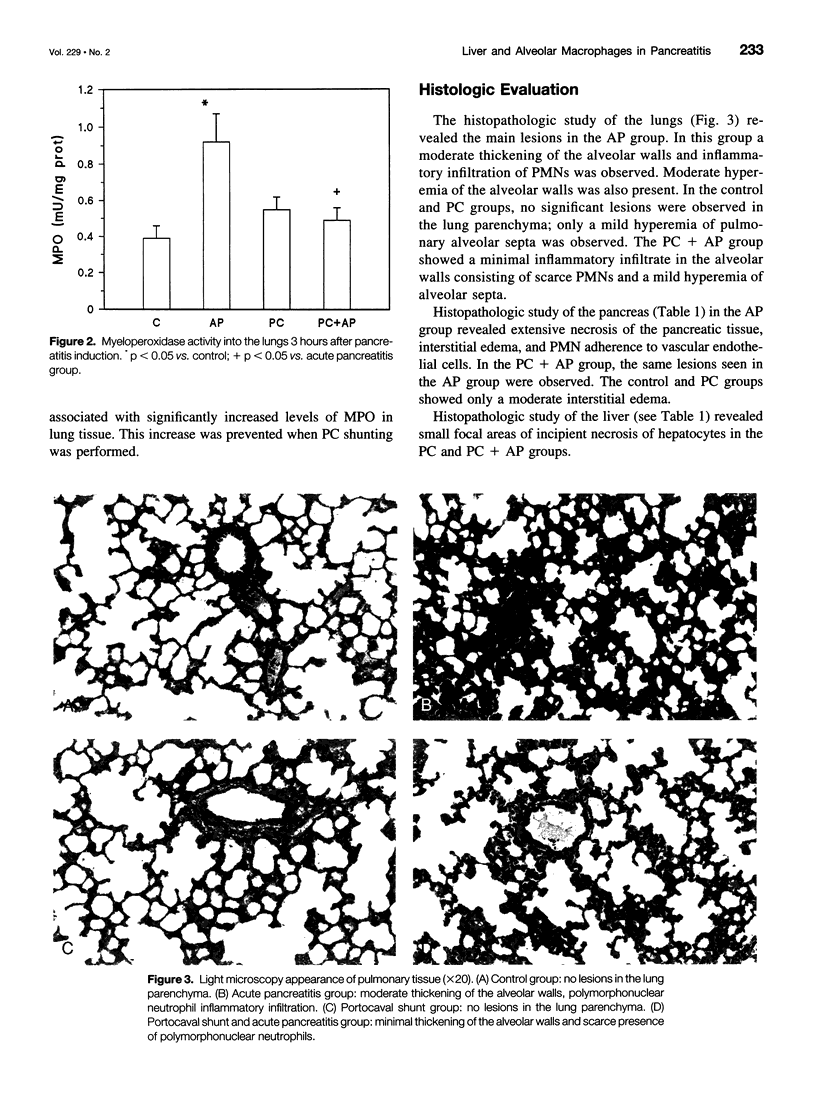
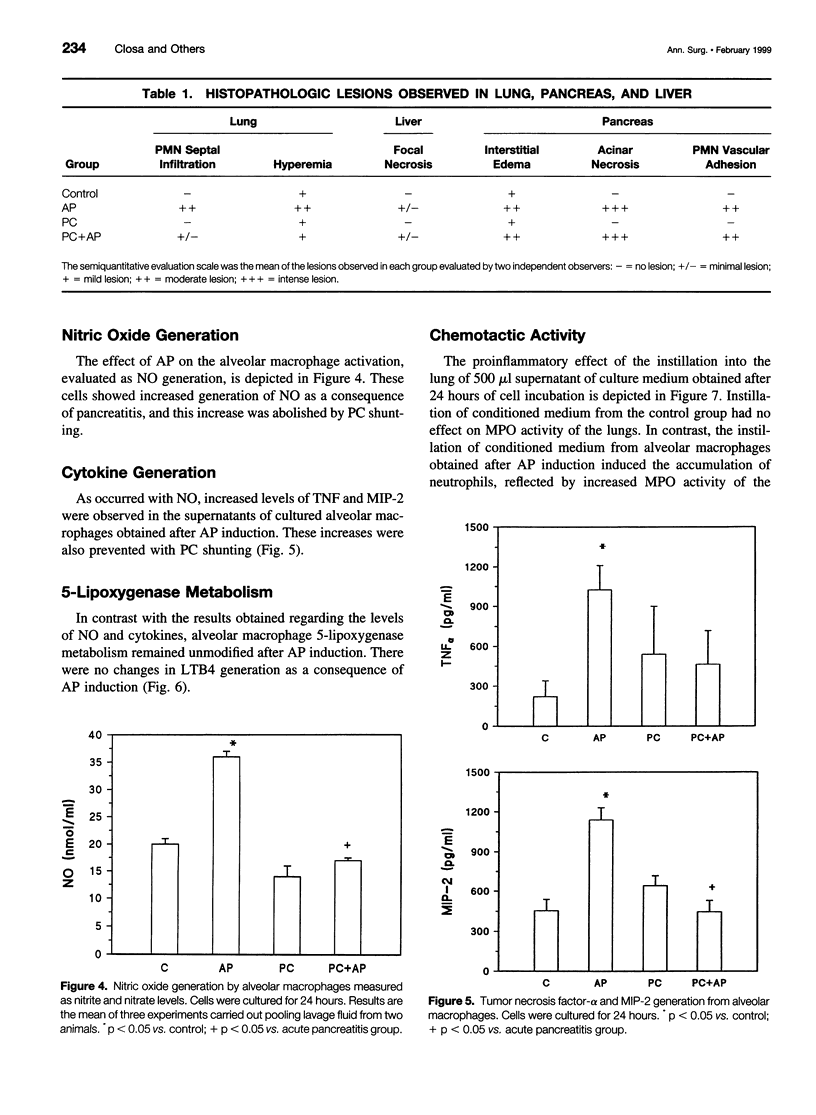
Abstract
OBJECTIVE: To evaluate (1) whether alveolar macrophages are activated as a consequence of acute pancreatitis (AP), (2) the implication of inflammatory factors released by these macrophages in the process of neutrophil migration into the lungs observed in lung injury induced by AP, and (3) the role of the liver in the activation of alveolar macrophages. SUMMARY BACKGROUND DATA: Acute lung injury is the extrapancreatic complication most frequently associated with death and complications in severe AP. Neutrophil infiltration into the lungs seems to be related to the release of systemic and local mediators. The liver and alveolar macrophages are sources of mediators that have been suggested to participate in the lung damage associated with AP. METHODS: Pancreatitis was induced in rats by intraductal administration of 5% sodium taurocholate. The inflammatory process in the lung and the activation of alveolar macrophages were investigated in animals with and without portocaval shunting 3 hours after AP induction. Alveolar macrophages were obtained by bronchoalveolar lavage. The generation of nitric oxide, leukotriene B4, tumor necrosis factor-alpha, and MIP-2 by alveolar macrophages and the chemotactic activity of supernatants of cultured macrophages were evaluated. RESULTS: Pancreatitis was associated with increased infiltration of neutrophils into the lungs 3 hours after induction. This effect was prevented by the portocaval shunt. Alveolar macrophages obtained after induction of pancreatitis generated increased levels of nitric oxide, tumor necrosis factor-alpha, and MIP-2, but not leukotriene B4. In addition, supernatants of these macrophages exhibited a chemotactic activity for neutrophils when instilled into the lungs of unmanipulated animals. All these effects were abolished when portocaval shunting was carried out before induction of pancreatitis. CONCLUSION: Lung damage induced by experimental AP is associated with alveolar macrophage activation. The liver mediates the alveolar macrophage activation in this experimental model.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Closa D., Rosello-Catafau J., Hotter G., Bulbena O., Fernandez-Cruz L., Gelpi E. Cyclooxygenase and lipoxygenase metabolism in sodium taurocholate induced acute hemorrhagic pancreatitis in rats. Prostaglandins. 1993 Apr;45(4):315–322. doi: 10.1016/0090-6980(93)90109-k. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994 Nov-Dec;20(6):473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- Dugernier T., Reynaert M. S., Deby-Dupont G., Roeseler J. J., Carlier M., Squifflet J. P., Deby C., Pincemail J., Lamy M., De Maeght S. Prospective evaluation of thoracic-duct drainage in the treatment of respiratory failure complicating severe acute pancreatitis. Intensive Care Med. 1989;15(6):372–378. doi: 10.1007/BF00261496. [DOI] [PubMed] [Google Scholar]
- Grönroos J. M., Nevalainen T. J. Increased concentrations of synovial-type phospholipase A2 in serum and pulmonary and renal complications in acute pancreatitis. Digestion. 1992;52(3-4):232–236. doi: 10.1159/000200958. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Caty M. G., Johnson K. J., Ward P. A. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989 Dec;210(6):740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Johnson K. J., Kunkel R. G., Morganroth M. L., Ward P. A. Pancreatitis-induced acute lung injury. An ARDS model. Ann Surg. 1988 Jul;208(1):71–77. doi: 10.1097/00000658-198807000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Meyers A. J., Gherini S. T., Beckmann A., Markison R. E., Churg A. M. Production of acute pulmonary injury by leukocytes and activated complement. Surgery. 1980 Jul;88(1):48–58. [PubMed] [Google Scholar]
- Hortelano S., Genaro A. M., Boscá L. Phorbol esters induce nitric oxide synthase activity in rat hepatocytes. Antagonism with the induction elicited by lipopolysaccharide. J Biol Chem. 1992 Dec 15;267(35):24937–24940. [PubMed] [Google Scholar]
- Kienast K., Knorst M., Müller-Quernheim J., Ferlinz R. Modulation of IL-1 beta, IL-6, IL-8, TNF-alpha, and TGF-beta secretions by alveolar macrophages under NO2 exposure. Lung. 1996;174(1):57–67. doi: 10.1007/BF00167951. [DOI] [PubMed] [Google Scholar]
- LEE S. H., FISHER B. Portacaval shunt in the rat. Surgery. 1961 Oct;50:668–672. [PubMed] [Google Scholar]
- McCord J. M., Gao B., Leff J., Flores S. C. Neutrophil-generated free radicals: possible mechanisms of injury in adult respiratory distress syndrome. Environ Health Perspect. 1994 Dec;102 (Suppl 10):57–60. doi: 10.1289/ehp.94102s1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J. G., Franz M. G., Fink G. S., Messina J., Fabri P. J., Gower W. R., Carey L. C. Decreased mortality of severe acute pancreatitis after proximal cytokine blockade. Ann Surg. 1995 Jun;221(6):625–634. doi: 10.1097/00000658-199506000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Horn J. K., Ong R., Goldstein I. M. Complement (C5)-derived chemotactic activity in serum from patients with pancreatitis. J Lab Clin Med. 1983 Jan;101(1):123–129. [PubMed] [Google Scholar]
- Steinberg W., Tenner S. Acute pancreatitis. N Engl J Med. 1994 Apr 28;330(17):1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- Trush M. A., Egner P. A., Kensler T. W. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem Toxicol. 1994 Feb;32(2):143–147. doi: 10.1016/0278-6915(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Tsukahara Y., Horita Y., Anan K., Morisaki T., Tanaka M., Torisu M. Role of nitric oxide derived from alveolar macrophages in the early phase of acute pancreatitis. J Surg Res. 1996 Nov;66(1):43–50. doi: 10.1006/jsre.1996.0370. [DOI] [PubMed] [Google Scholar]
- Yee J., Christou N. V. The local role of tumor necrosis factor alpha in the modulation of neutrophil function at sites of inflammation. Arch Surg. 1994 Dec;129(12):1249–1255. doi: 10.1001/archsurg.1994.01420360039004. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Nakagawa S., Koyama S., Kobayashi T., Homma T. Leukotriene B4 induces lung injury in the rabbit: role of neutrophils and effect of indomethacin. J Appl Physiol (1985) 1993 May;74(5):2174–2179. doi: 10.1152/jappl.1993.74.5.2174. [DOI] [PubMed] [Google Scholar]
- Zhou W., McCollum M. O., Levine B. A., Olson M. S. Role of platelet-activating factor in pancreatitis-associated acute lung injury in the rat. Am J Pathol. 1992 Apr;140(4):971–979. [PMC free article] [PubMed] [Google Scholar]



