Abstract
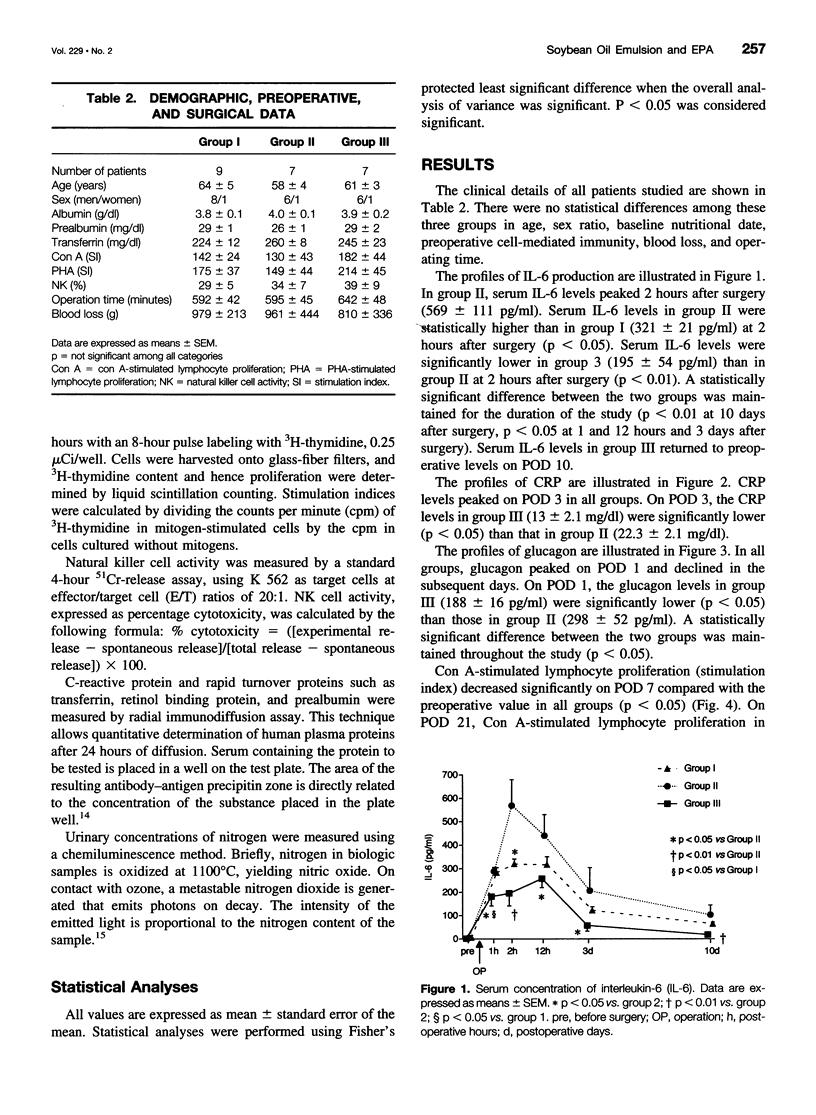
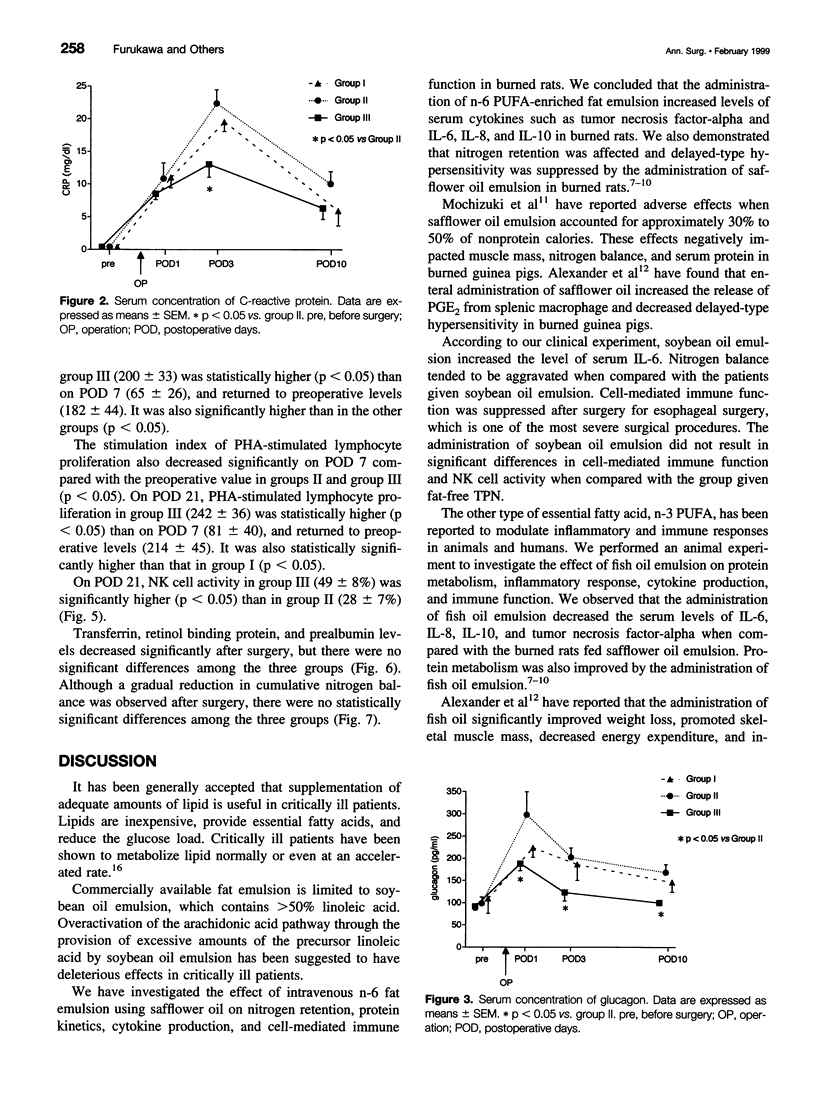
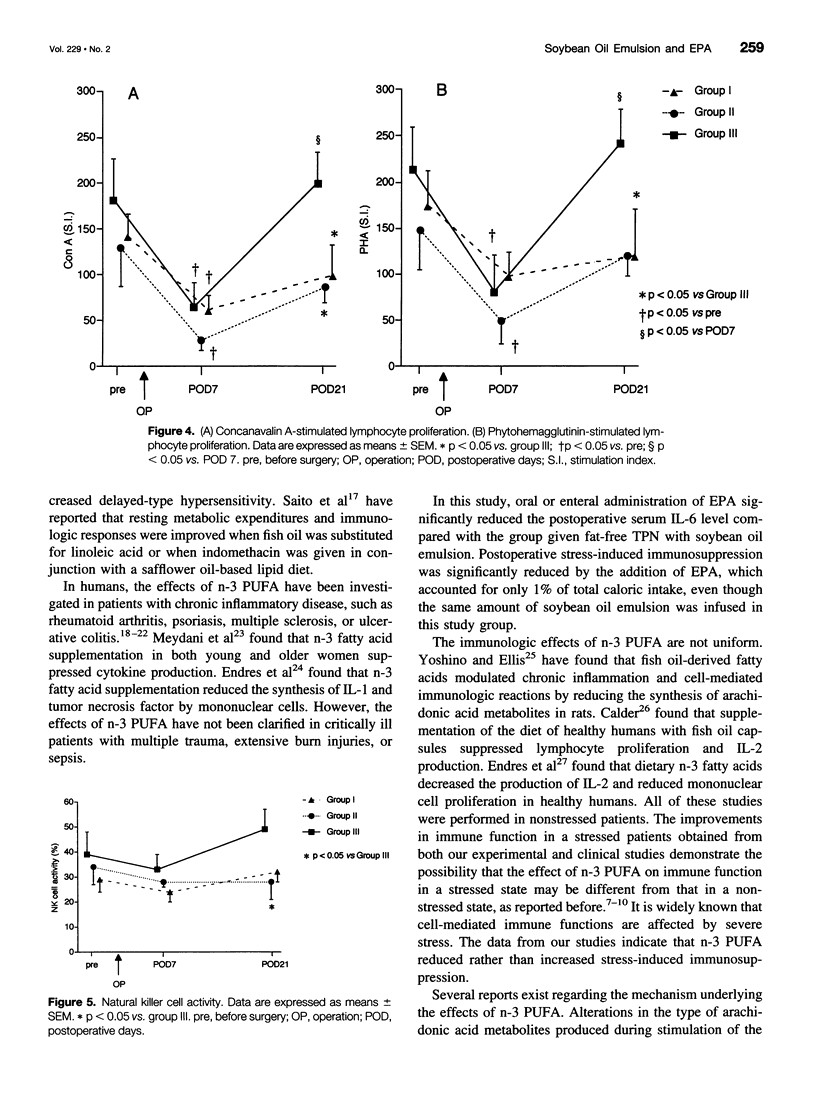
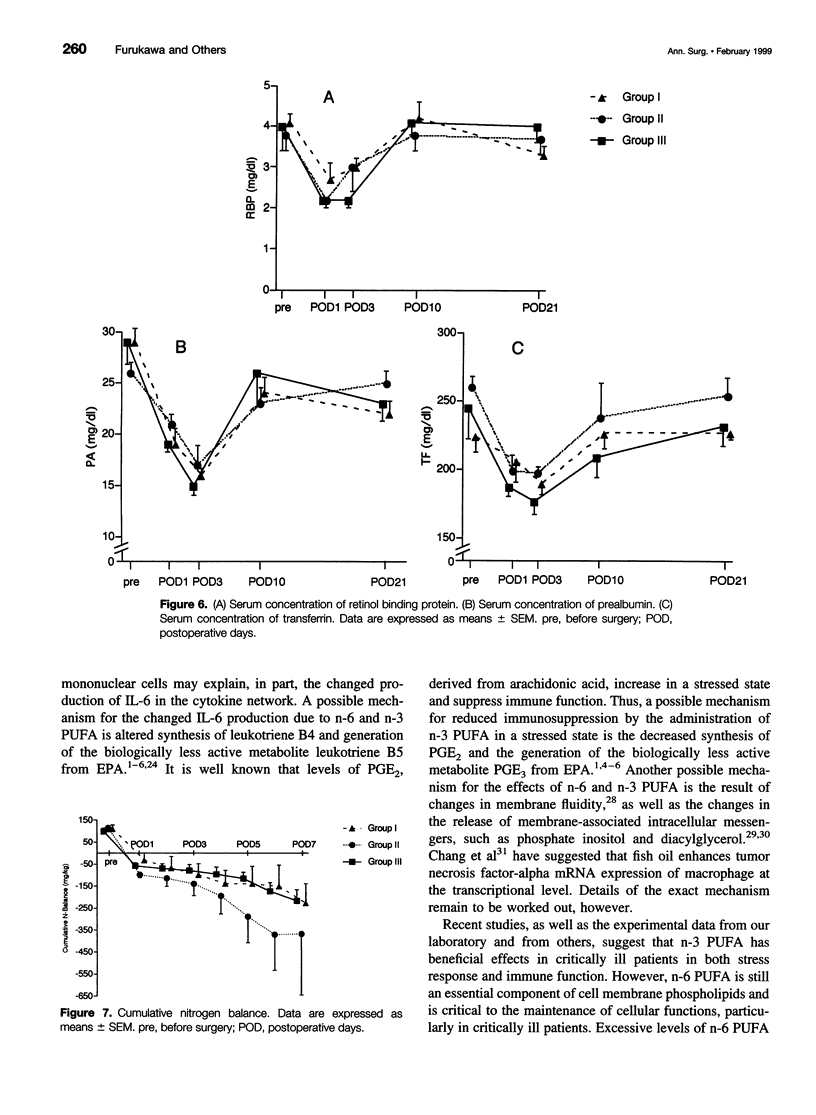
OBJECTIVE: To investigate the effects of soybean oil emulsion and oral or enteral administration of eicosapentaenoic acid (EPA) on stress response, cytokine production, protein metabolism, and immune function after surgery for esophageal cancer. SUMMARY BACKGROUND DATA: It has been reported that safflower oil, rich in n-6 polyunsaturated fatty acid (n-6 PUFA), affects the survival rate of septic animals and decreases the immune function. It has also been reported that the administration of fish oil, in contrast, reduces these stress responses and stress-induced immunosuppression. In humans, the effects of soybean oil emulsion and the administration of EPA on stress response and immune function after surgery have not been established. METHODS: Patients who underwent esophagectomy with thoracotomy were divided into three groups. Seven patients were fed by total parenteral nutrition (TPN) with soybean oil emulsion, which accounted for 20% of total calories. Seven patients were given oral or enteral administration of 1.8 g/day EPA, in addition to TPN with soybean oil emulsion. Nine patients served as the control group; these patients received fat-free TPN. Serum interleukin-6 (IL-6), C-reactive protein, concanavalin A (con A)- or phytohemagglutinin (PHA)-stimulated lymphocyte proliferation, natural killer cell activity, and stress hormones were measured. RESULTS: The postoperative level of serum IL-6 was significantly higher in the group receiving soybean oil emulsion than in the fat-free group. Oral or enteral supplementation of EPA with soybean oil emulsion significantly reduced the level of serum IL-6 compared with the patients receiving soybean oil emulsion. Con A- or PHA-stimulated lymphocyte proliferation decreased significantly on postoperative day 7 in all groups of patients. The supplementation of EPA with soybean oil emulsion significantly improved the lymphocyte proliferation and natural killer cell activity on postoperative day 21 compared with the group receiving soybean oil emulsion. CONCLUSIONS: Soybean oil emulsion amplifies, and the supplementation of EPA reduces, the stress response and stress-induced immunosuppression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Saito H., Trocki O., Ogle C. K. The importance of lipid type in the diet after burn injury. Ann Surg. 1986 Jul;204(1):1–8. doi: 10.1097/00000658-198607000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar T. R., Bankey P. E., Svingen B. A., Curran R. D., West M. A., Holman R. T., Simmons R. L., Cerra F. B. Fatty acid intake and Kupffer cell function: fish oil alters eicosanoid and monokine production to endotoxin stimulation. Surgery. 1988 Aug;104(2):343–349. [PubMed] [Google Scholar]
- Calder P. C. The effects of fatty acids on lymphocyte functions. Braz J Med Biol Res. 1993 Sep;26(9):901–917. [PubMed] [Google Scholar]
- Carpentier Y. A., Askanazi J., Elwyn D. H., Jeevanandam M., Gump F. E., Hyman A. I., Burr R., Kinney J. M. Effects of hypercaloric glucose infusion on lipid metabolism in injury and sepsis. J Trauma. 1979 Sep;19(9):649–654. doi: 10.1097/00005373-197909000-00002. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B., Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994 Apr 29;200(2):893–898. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- Chang H. R., Arsenijevic D., Vladoianu I. R., Girardier L., Dulloo A. G. Fish oil enhances macrophage tumor necrosis factor-alpha mRNA expression at the transcriptional level. Metabolism. 1995 Jun;44(6):800–805. doi: 10.1016/0026-0495(95)90196-5. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Kelley V. E., Georgilis K., Lonnemann G., van der Meer J. W., Cannon J. G., Rogers T. S., Klempner M. S., Weber P. C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- Endres S., Meydani S. N., Ghorbani R., Schindler R., Dinarello C. A. Dietary supplementation with n-3 fatty acids suppresses interleukin-2 production and mononuclear cell proliferation. J Leukoc Biol. 1993 Dec;54(6):599–603. [PubMed] [Google Scholar]
- Espersen G. T., Grunnet N., Lervang H. H., Nielsen G. L., Thomsen B. S., Faarvang K. L., Dyerberg J., Ernst E. Decreased interleukin-1 beta levels in plasma from rheumatoid arthritis patients after dietary supplementation with n-3 polyunsaturated fatty acids. Clin Rheumatol. 1992 Sep;11(3):393–395. doi: 10.1007/BF02207200. [DOI] [PubMed] [Google Scholar]
- Fowler K. H., McMurray D. N., Fan Y. Y., Aukema H. M., Chapkin R. S. Purified dietary n-3 polyunsaturated fatty acids alter diacylglycerol mass and molecular species composition in concanavalin A-stimulated murine splenocytes. Biochim Biophys Acta. 1993 Dec 2;1210(1):89–96. doi: 10.1016/0005-2760(93)90053-c. [DOI] [PubMed] [Google Scholar]
- Gallai V., Sarchielli P., Trequattrini A., Franceschini M., Floridi A., Firenze C., Alberti A., Di Benedetto D., Stragliotto E. Cytokine secretion and eicosanoid production in the peripheral blood mononuclear cells of MS patients undergoing dietary supplementation with n-3 polyunsaturated fatty acids. J Neuroimmunol. 1995 Feb;56(2):143–153. doi: 10.1016/0165-5728(94)00140-j. [DOI] [PubMed] [Google Scholar]
- Kinsella J. E., Lokesh B., Broughton S., Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990 Jan-Feb;6(1):24–62. [PubMed] [Google Scholar]
- Kremer J. M., Lawrence D. A., Petrillo G. F., Litts L. L., Mullaly P. M., Rynes R. I., Stocker R. P., Parhami N., Greenstein N. S., Fuchs B. R. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis Rheum. 1995 Aug;38(8):1107–1114. doi: 10.1002/art.1780380813. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Sethi T., Crea A. E., Peters W., Arm J. P., Horton C. E., Walport M. J., Spur B. W. Characterization of leukotriene B3: comparison of its biological activities with leukotriene B4 and leukotriene B5 in complement receptor enhancement, lysozyme release and chemotaxis of human neutrophils. Clin Sci (Lond) 1988 May;74(5):467–475. doi: 10.1042/cs0740467. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Meydani S. N., Endres S., Woods M. M., Goldin B. R., Soo C., Morrill-Labrode A., Dinarello C. A., Gorbach S. L. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991 Apr;121(4):547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Trocki O., Dominioni L., Ray M. B., Alexander J. W. Optimal lipid content for enteral diets following thermal injury. JPEN J Parenter Enteral Nutr. 1984 Nov-Dec;8(6):638–646. doi: 10.1177/0148607184008006638. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Surette M. E., Whelan J., Lu G., Hardard'ottir I., Kinsella J. E. Dietary n - 3 polyunsaturated fatty acids modify Syrian hamster platelet and macrophage phospholipid fatty acyl composition and eicosanoid synthesis: a controlled study. Biochim Biophys Acta. 1995 Mar 16;1255(2):185–191. doi: 10.1016/0005-2760(94)00206-e. [DOI] [PubMed] [Google Scholar]
- Søyland E., Lea T., Sandstad B., Drevon A. Dietary supplementation with very long-chain n-3 fatty acids in man decreases expression of the interleukin-2 receptor (CD25) on mitogen-stimulated lymphocytes from patients with inflammatory skin diseases. Eur J Clin Invest. 1994 Apr;24(4):236–242. doi: 10.1111/j.1365-2362.1994.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Ward M. W., Owens C. W., Rennie M. J. Nitrogen estimation in biological samples by use of chemiluminescence. Clin Chem. 1980 Aug;26(9):1336–1339. [PubMed] [Google Scholar]
- Yoshino S., Ellis E. F. Effect of a fish-oil-supplemented diet on inflammation and immunological processes in rats. Int Arch Allergy Appl Immunol. 1987;84(3):233–240. doi: 10.1159/000234429. [DOI] [PubMed] [Google Scholar]


