Abstract
In this prospective randomized clinical trial, we aimed to evaluate the safety and efficacy of endourethrotomy with holmium:yttrium‐aluminium‐garnet (HO:YAG) laser and compare the outcomes with the conventional cold‐knife urethrotomy. Fifty‐one male patients with single, iatrogenic, annular strictures of the urethra were randomly divided into two groups; 21 patients who underwent direct‐vision endoscopic urethrotomy with Ho:YAG laser (15 W; 1,200–1,400 mJ; 8–12 Hz) at 12 o'clock position (laser group) and 30 patients who underwent direct‐vision endoscopic urethrotomy with cold‐knife incision at 12 o'clock position (cold‐knife group). The results obtained were analyzed and compared at 3 months, 6 months, 9 months, and 12 months postoperatively by clinical evaluation, uroflowmetry, and retrograde urethrographies. Variables were compared among groups using Fisher's exact and Mann Whitney U tests. There were no differences between two groups in terms of patient age, preoperative Qmax value, stricture location, and length. Operative time was shorter in laser group (16.4 ± 8.04 minutes) when compared with cold‐knife group (23.8 ± 5.47 minutes) (p < 0.001). Recurrence‐free rate at 3 months was similar between two groups (p = 0.122). However, recurrence‐free rates at 6 months, 9 months, and 12 months were significantly higher in laser group when compared with cold‐knife group (p values were 0.045, 0.027, and 0.04, respectively). No intra‐ or postoperative complications were encountered. Use of Ho:YAG laser in the management of urethral stricture disease is a safe and effective method. In addition, it provides shorter operative time and lower recurrence rate when compared with the conventional technique.
Keywords: Cold knife, Ho:YAG, Internal urethrotomy, Laser, Urethral stricture disease
Introduction
Urethral stricture disease is defined as narrowing of the urethral lumen because of fibrosis, which occurs in urethral mucosa and surrounding tissues. The etiology could be congenital or idiopathic [1]. Scarred tissue formation does not occur in congenital strictures, therefore, congenital strictures are accepted as anomalies rather than true strictures. Urethral stricture disease might also occur as a result of inflammation. Trauma is another factor that leads to this clinical entity. On the other hand, posterior urethral strictures differ from anterior urethral strictures as they occur generally after partial or complete rupture of the urethra because of pelvic trauma. Posterior urethral injuries result after distention and traction type of trauma [2]. Anterior urethral injuries are usually isolated and related with horseback riding type of pelvic trauma.
Endoscopic urethrotomy was first introduced in 1973 by Sachse [3] with the use of a cold‐knife technique to incise those stricture segments. Several techniques are currently available for minimally invasive treatment of urethral strictures, including cold‐knife incision, electrocautery, and various types of laser incisions. Incision with the cold knife does not cause any thermal effect on surrounding tissues but should create mechanical injury that may lead to recurrence in long term. Incision with the electrocautery should cause significant thermal effect on healthy surrounding tissues resulting in recurrent strictures during follow‐up. Laser treatment modalities have gained popularity in the last two decades. Carbon dioxide, argon, potassium titanyl phosphate (KTP), neodymium (Nd):yttrium‐aluminium‐garnet (YAG) and holmium (Ho):YAG lasers have been used in the treatment of urethral stricture disease. Recently, the Ho:YAG laser became the primary choice in endourology practice. It is a kind of a pulsed far infrared ray with a wavelength of 2,100 nm. It shows superior capabilities in incisions and vaporization with less thermal effect compared with electrocautery and other laser types.
In our study, we aimed to evaluate the safety and efficacy of endourethrotomy with Ho:YAG laser. To the best of our knowledge, this is the first prospective, randomized clinical trial that compared the outcomes of Ho:YAG laser urethrotomy with the conventional cold‐knife technique.
Materials and methods
The study was approved by our institutional review board. All the patients gave us a signed, written informed consent form before performing surgery. A total of 51 male patients who underwent optic urethrotomy for single, iatrogenic, annular strictures of the urethra were enrolled in the study. The patients were randomized into two groups as the Ho:YAG laser (laser group) and cold‐knife groups (cold‐knife group). The randomization was performed by a “random number table,” allocating 21 men to the laser treatment group and 30 to the cold‐knife treatment group. Laser group comprised 21 patients who underwent direct‐vision endoscopic urethrotomy with Ho:YAG laser (15 W; 1,200–1,400 mJ; 8–12 Hz) at 12 o'clock position (Fig. 1). Cold‐knife group comprised 30 patients who underwent direct‐vision endoscopic urethrotomy with cold‐knife incision at 12 o'clock position.
Figure 1.
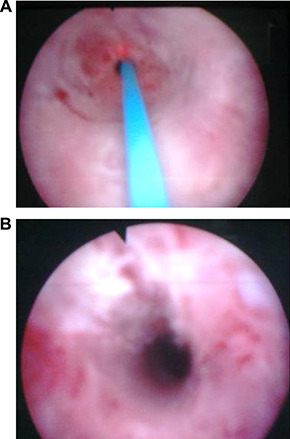
Images (A) before and (B) after laser urethrotomy.
All patients were preoperatively evaluated with following parameters: full personal and family history, physical examination, routine blood analysis (complete blood counts and serum biochemical analysis), urine analysis and culture, uroflowmetric assessment.
Stricture localization and length of the stricture segment were identified during direct visualization with optical urethrotome. A 5 French (F) ureteral catheter was used to measure the length of the stricture segment. In addition, operative time was recorded. Operative time was described as the time interval beginning with insertion of optical urethrotome from external urethral meatus, continuing with the treatment of stricture site either by laser or cold‐knife, and ending with the removal of urethrotome from external urethral meatus.
All patients were reevaluated at first month and 3 months, 6 months, 9 months, and 12 months postoperatively. During follow‐up, patients were assessed with detailed physical examination, urine analysis and culture, uroflowmetry (repeated if voided volume was <150 mL), and retrograde urethrography if obstructive symptoms were developed or maximum flow rate during uroflowmetric assessment was 10 mL/sec or lower.
The procedure was accepted as successful if the patient did not report any voiding difficulty, the maximum flow rate (Qmax) was greater than 10 mL/sec with no or insignificant residual urine (<100 mL). All patients were catheterized for 24–48 hours postoperatively (16F silicone urethral catheter) and received single dose of 1 g ceftriaxone intravenously 30 minutes before the operation. Postoperatively, 500 mg cefuroxime axetil (twice a day) was prescribed for 7 days.
Exclusion criteria were strictures greater than 20 mm in length, multiple (>1 site), congenital, postradical prostatectomy strictures, or strictures which recurred more than once (>1 recurrence). Patients who were lost during follow‐up were also excluded from the study.
Surgical technique
All the procedures have been performed by one surgeon (M. A.). Surgical procedures were performed under general or regional anesthesia. Patients were placed in a lithotomy position and stricture sites were examined using a 20F cystoscope. After the measurement of stricture length via 5F ureteral catheter, a 0.035 in. hydrophilic glide wire was inserted into the bladder through cystoscope. The cystoscope was removed out from urethra leaving the glide wire inside. Then, with a 22F cystoscope, stricture site was incised at 12 o'clock position using a 500‐u3 fiber at 15 W laser power (1,200–1,400 mJ and 8–12 Hz frequency) (1, 2). In patients randomized to cold‐knife group, stricture site was incised at 12 o'clock position using a cold knife via 21F urethrotome. After complete incision of stricture site and achieving easy passage of cystoscope or urethrotome into the bladder, an indwelling 16F silicone catheter was left in place for 24–48 hours.
Figure 2.

Recurrence‐free rates for 12 months. Although statistically insignificant, please note that most recurrences in cold‐knife group were seen during first 3 months postoperatively [*statistically significant p value (Fisher's exact test)].
Statistics
Statistical analysis was performed using SSPS Version 11.0 (SPSS, Chicago, IL, USA). Numerical variables, such as demographic data, operative time, and Qmax values, were assessed by using Mann‐Whitney U test. Categorical variables, such as stricture localization, stricture type (primary/secondary), recurrence, and recurrence‐free rates, were analyzed by Fisher's exact test. A p value less than 0.05 was accepted as statistically significant.
Results
Twenty‐one (41%) of 51 cases underwent Ho:YAG laser urethrotomy (laser group) for urethral stricture disease, whereas 30 (59%) cases underwent classic (cold knife) internal urethrotomy (cold‐knife group). Mean age for the whole study population was 61.41 ± 12.86 years (range: 24–85 years). Iatrogenic bladder neck contraction after transurethral resection of the prostate was the etiology for posterior urethral strictures in all cases. Similarly, for anterior urethral strictures, causative factor was iatrogenic reasons (such as history of prolonged or traumatic urethral catheterization). Stricture site was bulbous urethra in all patients who had anterior urethral strictures.
There were no statistically significant differences between two groups in terms of patient age, preoperative Qmax value, and stricture location and length (Table 1). However, there was a statistically significant difference when both groups were compared in terms of stricture type (p = 0.043, Fisher's exact test). Recurrent strictures (secondary) were greater in laser group (Table 1).
Table 1.
Characteristics in study groups and comparability of groups treated
| Parameters | Laser group (n = 21) | Cold‐knife group (n = 30) | p |
|---|---|---|---|
| Age (y) | 63.85 ± 7.98 (49–79) | 59.70 ± 15.29 (24–85) | 0.774 a |
| Primary/secondary (recurrent), n (%) | 15 (71.4)/6 (28.6) | 28 (93.3)/2 (6.7) | 0.043 b |
| Anterior/posterior, n (%) | 13 (61.9)/8 (38.1) | 20 (66.7)/10 (33.3) | 0.477 b |
| Length of stricture segment (mm) | 11.09 ± 3.28 (6–18) | 12.3 ± 2.98 (10–19) | 0.159 a |
| Operative time (min) | 16.42 ± 8.04 (7–35) | 23.83 ± 5.47 (15–40) | <0.001 a |
| Preoperative Qmax value (mL/sec) | 4.52 ± 1.98 | 4.73 ± 1.47 | 0.449 a |
| Recurrence/no recurrence, n (%) | 4 (19)/17 (81) | 14 (46.7)/16 (53.3) | 0.040 b |
Qmax = maximum flow rate per second.
Mann‐Whitney U test.
Fisher's exact test.
Operative time was shorter in laser group (16.42 ± 8.04 minutes) when compared with cold‐knife group (23.83 ± 5.47 minutes) (p < 0.001, Mann Whitney U test). Recurrence rates for the laser and cold‐knife groups were 19% and 46.7%, respectively (Table 1). Recurrence‐free rate at 3 months was similar between two groups (p = 0.122, Fisher's exact test). However, recurrence‐free rates at 6 months, 9 months, and 12 months were significantly higher in laser group when compared with cold‐knife group (p values were 0.045, 0.027, and 0.04, respectively) (Fig. 2).
No statistically significant differences were seen between two groups when preoperative, postoperative first month, 6‐month and 12‐month Qmax values were compared (data not shown).
No intra‐ or postoperative complications, such as hemorrhage, false route, bacteremia, urinary retention, epididymitis, were encountered in any group.
Discussion
Currently, laser is one of the most preferred technological modalities in endoscopic urologic surgery. Less hemorrhage and shorter hospitalization time are the most important advantages in laser surgery [4]. Choice of the surgical technique depends on experience of surgeon and equipment of the hospital. Because of high recurrence risk in urethral stricture diseases, deciding for the type of surgical intervention remains a critically important issue for endourologists. High recurrence rates, reaching up to 80%, were reported in literature [5]. Those high rates forced clinicians to alternative treatment methods, such as various types of laser applications in urethral stricture disease management. Nd:YAG, KTP, argon, excimer, diode lasers, and finally Ho:YAG laser have been used in endoscopic treatment of urethral stricture disease [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]]. Bloiso et al. [9] documented 67% recurrence rate with Nd:YAG laser after 1‐year follow‐up. However, Perkash [5] and others reported a 93% success over a follow‐up of 28 months by using contact chisel‐crystal fired Nd:YAG laser. Unfortunately, most authors could not reach this high success rate [[12], [13], [14], [15], [16]].
Kamal [17] treated 22 urethral stricture patients by using a 400–600 μm diode laser in direct‐contact mode. After a mean follow‐up of 26.7 months, no recurrence was observed in 11 of 14 cases with previously untreated strictures (primary) (21.4% recurrence rate). However, seven of the eight cases (87.5%) with a recurrent stricture after previous internal cold‐knife urethrotomy (secondary) had a recurrence after laser urethrotomy during follow‐up period. As Dr. Kamal [17] emphasized in the article, deep fibrotic urethral tissue formation, which decreases the long‐term success of the treatment, is more pronounced in secondary cases. So, it is not surprising to observe significantly higher recurrence rates in secondary cases. The number of secondary strictures in our study was significantly higher in laser group (p = 0.043, Table 1). Despite this difference, significantly lower recurrence and higher recurrence‐free rates were noticed in laser group when compared with cold‐knife group. Nevertheless, additional prospective randomized trials with similar primary and secondary stricture cases would better identify the difference between the outcomes in laser and conventional urethrotomy groups.
Development of restenosis after laser applications is mainly affected by the wavelength, tissue absorption, and thermal effects of the laser energy. Deepest tissue penetration was demonstrated with Nd:YAG and diode lasers (4–5 mm). KTP and argon lasers have a 1–3 mm tissue penetration. Ho:YAG laser has the lowest tissue penetration (less than 0.5 mm). For any treatment with laser in narrow luminal organs, such as urethra, selection of laser types with minimum tissue absorption, such as Ho:YAG, may decrease scarred tissue reactions over treated area. Several studies have documented less scarred tissue formation at incision site with laser surgery when compared with cold knife or electrocautery [18]. During incision with a laser in urethral stricture disease, scarred tissue was also evaporated, so extirpation of the fibrous tissue by direct contact of the laser fiber could be achieved even when low power energy was used [10].
In a study by Montorsi et al. [4], Ho:YAG laser was used for the enucleation of prostate and less hemorrhage, shorter catheterization time, and hospital stay were reported with Ho:YAG when compared with transurethral resection of the prostate. Naturally, similar results should be expected for treatment of urethral strictures with Ho:YAG laser. In our trial, no complications were encountered in any study groups. Therefore, we accepted Ho:YAG laser as safe and reliable as conventional cold‐knife urethrotomy. In a study by Kamp et al. [19], 32 male patients with symptomatic urethral strictures were treated with Ho:YAG laser urethrotomy at 12 o'clock position. As in our study, laser energy used in this study was 1,200–1,400 mj. Although, laser energy was set on a frequency of 10–13 Hz in the study by Kamp et al. [19], we preferred a lower frequency, 8–12 Hz, to minimize deep tissue penetration and coagulation on urethra. This low setting may also have contributed to the success of treatment by preventing thermal injury on surrounding healthy tissues, thereby decreasing reactive fibrous tissue formation. The success rates were comparable (In the study by Kamp, overall success rate was 70% after a mean 27‐month follow‐up period and in present study, it was 81% after a 12‐month follow‐up period).
“Time to recurrence” is also an important parameter in urethral stricture disease. In the cold‐knife group, 8 of 14 (57.1%) recurrences appeared within the first 3 months, whereas in the laser group, 2 of 4 (50%) recurrences were demonstrated within the first 3 months. Within the 3–6‐months period during follow‐up, one new case in the laser group and four new cases in the cold‐knife group were diagnosed. Within the 6–9‐months period and 9–12 months‐period, the number of new recurrent cases were zero and one for the laser group, and one and one for the cold‐knife group, respectively. So, for our study population, we can say that the cold‐knife treated cases tended to recur within the first 3 months or 6 months postoperatively when compared with laser‐treated patients.
It is proposed to perform internal urethrotomies between two corpus cavernosum at 12 o'clock position to avoid hemorrhage. However, single‐site incision is not enough for some strictures and additional incisions at 6 o'clock position may be necessary. Alternatively, incisions at 10 o'clock position and 2 o'clock position were also suggested [20]. Currently, among endourologists, most popular method is to incise at 12 o'clock position or at the location where maximal scarred tissue formation occurred [19]. We performed incisions in both groups at 12 o'clock position and no corpus cavernosum injury was observed in any of our patients.
Kamp et al. [19] reported that the stricture site, operative time, and the duration of catheterization had no impact on the recurrence of stricture or success of treatment. However, previous urethrotomy and stricture length were found to be significant risk factors for the recurrence. In our study, operative time was significantly lower in laser group. We hypothesize that shorter operative time in laser group might participate in low recurrence rate observed. Thus, prevention of prolonged operative time during urethrotomy surgery should increase the success of treatment.
Duration of urethral catheterization after endoscopic incision of urethral strictures is still a controversial issue. In the literature, this duration ranges from 24 hours up to 6 weeks [[5], [12], [20]]. But, postoperatively prolonged urethral catheterization is accepted as the most important risk factor for urinary tract infections [21]. Those infections may result in sepsis, prolonged hospital stay, increased costs, and mortality. To avoid related complications, we performed urethral catheterization for a maximum of 48 hours in our study.
In recent years, the choice of prophylactic antibiotics has gained importance because of increased antimicrobial resistance among the whole world. Resistance rate to ciprofloxacin reached up to 20% for Escherichia coli, Pseudomonas, and Klebsiella [22]. Kashanian et al. [23] evaluated 10,000 E coli urine cultures and reported a 24% resistance prevalence for ciprofloxacin. Wagenlehner et al. [24] found 12% ciprofloxacin resistance even after single dose of prophylactic ciprofloxacin administration. When compared with ciprofloxacin, lower rates of cephalosporin resistance were reported [22]. Therefore, for prophylaxis, we administered a single dose of cephalosporin 30 minutes before surgery and continued with oral sefuroxime axetil postoperatively.
In conclusion, use of Ho:YAG laser in the management of urethral stricture disease is a safe and effective method. It also provides shorter operative time and lower recurrence rate when compared with the conventional technique. Nevertheless, randomized trials with large series and longer follow‐up period are mandatory to give a discrete conclusion.
References
- [1]. Jordan G.H., Devine P.C.. Management of urethral stricture disease. Urol Clin North Am. 1988; 15: 277–289. [Google Scholar]
- [2]. Gallentine M.L., Morey A.F.. FACS: imaging of the male uretra for stricture disease. Urol Clin North Am. 2002; 29: 361–372. [DOI] [PubMed] [Google Scholar]
- [3]. Sachse H.. Die transurethrale scharfe Schlitzung der Harnrohrenstriktur mit einem Sichturethrotom. Verhandl Deutsches Gesell Urol. 1973; 25: 143–146. [Google Scholar]
- [4]. Montorsi F., Naspro R., Salonia A., Suardi N., Briganti A., Zanoni M., et al. Holmium laser enucleation versus transurethral resection of the prostate: Results from a 2‐ center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol. 2004; 172: 1926–1929. [DOI] [PubMed] [Google Scholar]
- [5]. Perkash I.. Ablation of urethral strictures using contact chisel crystal fıring neodymium:YAG laser. J Urol. 1997; 157: 809–813. [PubMed] [Google Scholar]
- [6]. Pansadoro V., Emilliozzi P.. Internal urethrotomy in the management of anterior urethral strictures: long‐term follow up. J Urol. 1996; 156: 73–75. [PubMed] [Google Scholar]
- [7]. Bulow H., Frohnuller H.G.. Transurethral laser urethrotomy in man: preliminary report. J Urol. 1979; 121: 286–287. [DOI] [PubMed] [Google Scholar]
- [8]. Smith J.A. Jr., Dixon J.A.. Neodymium:YAG laser treatment of benign urethral strictures. J Urol. 1984; 131: 1080–1081. [DOI] [PubMed] [Google Scholar]
- [9]. Bloiso G., Warner R., Cohen M.. Treatment of urethral diseases with neodymium:YAG laser. J Urol. 1988; 32: 106–110. [DOI] [PubMed] [Google Scholar]
- [10]. Smith J.A. Jr.. Treatment of benign urethral strictures using a sapphire tipped neodymium:YAG laser. J Urol. 1989; 142: 1221–1222. [DOI] [PubMed] [Google Scholar]
- [11]. Dogra P.N., Argon M., Rajeev T.P.. Core‐through urethrotomy with neodymium: YAG laser for post‐traumatic obliterative strictures of the bulbomembranous urethra. J Urol. 1999; 161: 81–84. [DOI] [PubMed] [Google Scholar]
- [12]. Vicente J., Salvador J., Caffaratti J.. Endoscopic urethrotomy versus urethrotomy plus Nd:YAG laser in the treatment of urethral stricture. Eur Urol. 1990; 18: 166–168. [DOI] [PubMed] [Google Scholar]
- [13]. Turek P.J., Mallory T.R., Cendron M., Carpiniello V.L., Wein A.J.. KTP‐532 laser ablation of urethral strictures. Urology. 1992; 40: 330–334. [DOI] [PubMed] [Google Scholar]
- [14]. Becker H.C., Miler J., Noske H.D., Klask J.P., Weidrer W.. Transurethral laser uretrotomy with the argon laser: experience with 900 urethrotomies in 450 patients from 1978 to 1993. Urol Int. 1995; 55: 150–153. [DOI] [PubMed] [Google Scholar]
- [15]. Adkins W.C.. Argon laser treatment of urethral stricture and vesical neck contracture. Laser Surg Med. 1988; 8: 600–603. [DOI] [PubMed] [Google Scholar]
- [16]. Baur H., Schneider W., Altwein J.E.. Treatment of recurrent urethral strictures by photoablation with Excimer laser. BAUS Abstract Book, 1992; 118
- [17]. Kamal B.A.. The use of the diode laser for treating urethral strictures. BJU Int. 2001; 87: 831–833. [DOI] [PubMed] [Google Scholar]
- [18]. Jordan G.H., Schlossberg S.M., Devine C.J.. Surgery of the penis and urethra. In Walsh P.C., Retik A.B., eds. Campbell s urology. 7th ed. Philadelphia: Saunders. 1998, Vol. 6, 3347, Chapter 107. [Google Scholar]
- [19]. Kamp S., Knoll T., Osman M.M., Köhrmann K.U., Michel M.S., Alken P.. Low‐power holmium: YAG laser urethrotomy for treatment of urethral strictures: functional outcome and quality of life. J Endourol. 2006; 20: 38–41. [DOI] [PubMed] [Google Scholar]
- [20]. Motsouka K., Inoue M., Lida S., Tomiyasu K., Noda S.. Endoscopic antegrade laser incision in the treatment of urethral stricture. Urology. 2002; 60: 968–972. [DOI] [PubMed] [Google Scholar]
- [21]. Elpern E.H., Killen K.I., Ketchem M., Wiley A., Patel G., Lateef O.. Reducing the use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009; 18: 535–541. [DOI] [PubMed] [Google Scholar]
- [22]. Das Gupta R., Sullivan R., French G., O'Brien T.. Evidence‐based prescription of antibiotics in urology: A 5‐year review of microbiology. BJU Int. 2009; 104: 760–764. [DOI] [PubMed] [Google Scholar]
- [23]. Kashanian J., Hakimian P., Blute M. Jr., Wong J., Khanna H., Wise G., et al. Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int. 2008; 102: 1634–1637. [DOI] [PubMed] [Google Scholar]
- [24]. Wagenlehner F., Stöwer‐Hoffmann J., Schneider‐Brachert W., Naber K.G., Lehn N.. Influence of a prophylactic single dose of ciprofloxacin on the level of resistance of E. Coli to fluoroquinolones in urology. Int J Antimicrob Agents. 2000; 15: 207–211. [DOI] [PubMed] [Google Scholar]


