Abstract
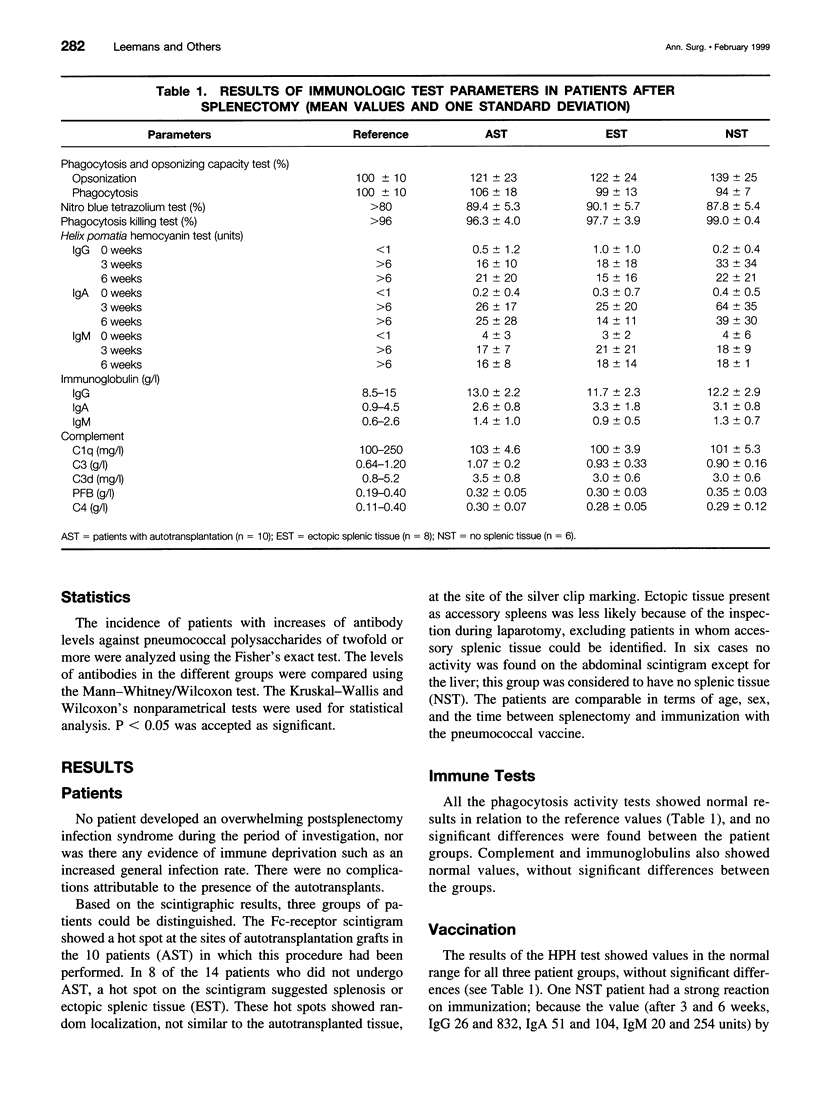
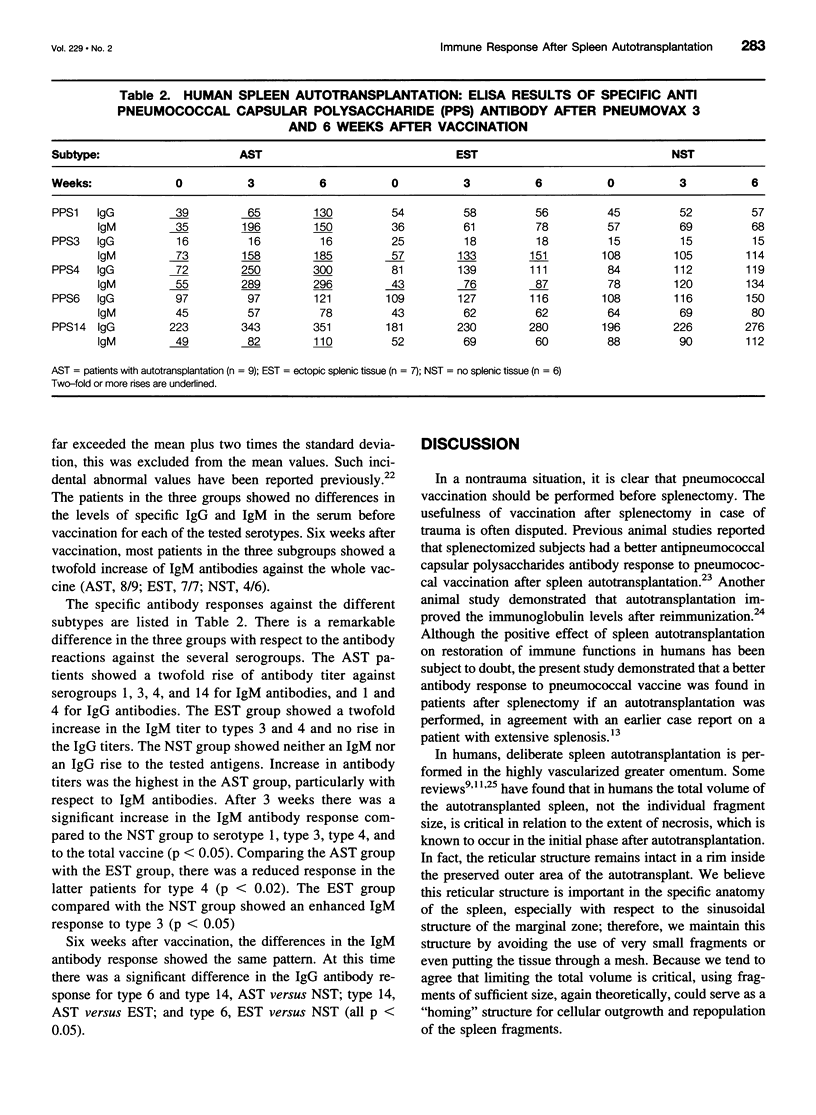
OBJECTIVE: To evaluate features of general immune function, in particular the restoration of the humoral immune response to pneumococcal capsular polysaccharides, in humans undergoing a spleen autotransplantation after splenectomy because of trauma. SUMMARY BACKGROUND DATA: After splenectomy, patients have an increased risk of overwhelming infection or sepsis involving encapsulated bacteria such as pneumococci. The value of human spleen autotransplantation after splenectomy because of trauma has long been questioned. Mononuclear phagocyte system function appeared to be similar to that in splenectomized persons. The presence of specific antipneumococcal antibodies would allow other parts of the mononuclear phagocyte system, such as those in the liver, to phagocytose opsonized bacteria. METHODS: Ten consecutive patients undergoing splenectomy followed by autotransplantation were compared with the next 14 consecutive patients undergoing splenectomy alone. After a minimum of 6 months, the patients were vaccinated with 23-valent pneumococcal vaccine. Blood samples were taken at the time of vaccination and after 3 and 6 weeks for antipneumococcal capsular polysaccharides IgM and IgG enzyme-linked immunosorbent assay against types 3, 4, 6, 9, 14, and 23. Splenic regrowth was evaluated by scintigraphy. RESULTS: Surprisingly, several of the nonautotransplanted patients showed scintigraphic activity, indicating the presence of either accessory spleens or traumatic seeding (splenosis). Significant antibody titer increases (more than twofold) were found for both IgM and IgG in the autotransplanted patients. Splenectomized-only patients showed no significant increase in Ig levels in patients without splenic regrowth and partial improvement in patients with splenosis/accessory spleens. CONCLUSIONS: Considering this significant antipneumococcal antibody increase, spleen autotransplants can be expected to permit an adequate humoral response to pneumococcal infections and presumably also to other TI-2 antigens, and to protect against overwhelming postsplenectomy infection or sepsis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr N. J., Turk E. P. The histological features of splenosis. Histopathology. 1992 Dec;21(6):549–553. doi: 10.1111/j.1365-2559.1992.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Cohn D. A., Schiffman G. Immunoregulatory role of the spleen in antibody responses to pneumococcal polysaccharide antigens. Infect Immun. 1987 Jun;55(6):1375–1380. doi: 10.1128/iai.55.6.1375-1380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantopoulos A., Najjar V. A., Wish J. B., Necheles T. H., Stolbach L. L. Defective phagocytosis due to tuftsin deficiency in splenectomized subjects. Am J Dis Child. 1973 May;125(5):663–665. doi: 10.1001/archpedi.1973.04160050017004. [DOI] [PubMed] [Google Scholar]
- Dahl M., Håkansson L., Kreuger A., Olsen L., Nilsson U., Venge P. Polymorphonuclear neutrophil function and infections following splenectomy in childhood. Scand J Haematol. 1986 Aug;37(2):137–143. doi: 10.1111/j.1600-0609.1986.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Fasching M. C., Cooney D. R. Reimmunization and splenic autotransplantation: a long-term study of immunologic response and survival following pneumococcal challenge. J Surg Res. 1980 May;28(5):449–459. doi: 10.1016/0022-4804(80)90109-2. [DOI] [PubMed] [Google Scholar]
- Hathaway J. M., Harley R. A., Self S., Schiffman G., Virella G. Immunological function in post-traumatic splenosis. Clin Immunol Immunopathol. 1995 Feb;74(2):143–150. doi: 10.1006/clin.1995.1021. [DOI] [PubMed] [Google Scholar]
- Hebert J. C., Fisher J. M., Ershler W. B. Serum antibody responses to pneumococcal vaccine after splenic autotransplantation. J Trauma. 1989 Mar;29(3):355–359. doi: 10.1097/00005373-198903000-00013. [DOI] [PubMed] [Google Scholar]
- Holdsworth R. J., Irving A. D., Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg. 1991 Sep;78(9):1031–1038. doi: 10.1002/bjs.1800780904. [DOI] [PubMed] [Google Scholar]
- Holdsworth R. J. Regeneration of the spleen and splenic autotransplantation. Br J Surg. 1991 Mar;78(3):270–278. doi: 10.1002/bjs.1800780305. [DOI] [PubMed] [Google Scholar]
- Johansen K. S. Nitroblue tetrazolium slide test. Use of the phorbol-myristate-acetate-stimulated NBT-reduction slide test for routine and prenatal detection of chronic granulomatous disease and diagnosis of heterozygous carriers. Acta Pathol Microbiol Immunol Scand C. 1983 Dec;91(6):349–354. [PubMed] [Google Scholar]
- Kiroff G. K., Hodgen A. N., Drew P. A., Jamieson G. G. Lack of effect of splenic regrowth on the reduced antibody responses to pneumococcal polysaccharides in splenectomized patients. Clin Exp Immunol. 1985 Oct;62(1):48–56. [PMC free article] [PubMed] [Google Scholar]
- Knowles D. J., Weston B. J. A simple rapid technique to measure neutrophil or serum bactericidal activity. J Immunol Methods. 1984 Sep 4;72(2):411–420. doi: 10.1016/0022-1759(84)90009-7. [DOI] [PubMed] [Google Scholar]
- Kuypers T. W., Eckmann C. M., Weening R. S., Roos D. A rapid turbidimetric assay of phagocytosis and serum opsonizing capacity. J Immunol Methods. 1989 Nov 13;124(1):85–94. doi: 10.1016/0022-1759(89)90189-0. [DOI] [PubMed] [Google Scholar]
- Lanng Nielsen J., Saksø P., Hanberg Sørensen F., Hvid Hansen H. Demonstration of splenic functions following splenectomy and autologous spleen implantation. Acta Chir Scand. 1984;150(6):469–473. [PubMed] [Google Scholar]
- Leemans R., Beekhuis H., Timens W., The T. H., Klasen H. J. Fc-receptor function after human splenic autotransplantation. Br J Surg. 1996 Apr;83(4):543–546. doi: 10.1002/bjs.1800830436. [DOI] [PubMed] [Google Scholar]
- Mizrahi S., Bickel A., Haj M., Lunski I., Shtamler B. Posttraumatic autotransplantation of spleen tissue. Arch Surg. 1989 Jul;124(7):863–865. doi: 10.1001/archsurg.1989.01410070123025. [DOI] [PubMed] [Google Scholar]
- Najjar V. A., Nishioka K. "Tuftsin": a natural phagocytosis stimulating peptide. Nature. 1970 Nov 14;228(5272):672–673. doi: 10.1038/228672a0. [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Igo R. P. The NBT slide test: a simple screening method for detecting chronic granulomatous disease and female carriers. J Pediatr. 1973 Jul;83(1):77–82. doi: 10.1016/s0022-3476(73)80316-6. [DOI] [PubMed] [Google Scholar]
- Pabst R., Westermann J., Rothkötter H. J. Immunoarchitecture of regenerated splenic and lymph node transplants. Int Rev Cytol. 1991;128:215–260. doi: 10.1016/s0074-7696(08)60500-8. [DOI] [PubMed] [Google Scholar]
- Patel J., Williams J. S., Naim J. O., Hinshaw J. R. Protection against pneumococcal sepsis in splenectomized rats by implantation of splenic tissue into an omental pouch. Surgery. 1982 Jun;91(6):638–641. [PubMed] [Google Scholar]
- Pearson H. A., Johnston D., Smith K. A., Touloukian R. J. The born-again spleen. Return of splenic function after splenectomy for trauma. N Engl J Med. 1978 Jun 22;298(25):1389–1392. doi: 10.1056/NEJM197806222982504. [DOI] [PubMed] [Google Scholar]
- Pimpl W., Dapunt O., Kaindl H., Thalhamer J. Incidence of septic and thromboembolic-related deaths after splenectomy in adults. Br J Surg. 1989 May;76(5):517–521. doi: 10.1002/bjs.1800760528. [DOI] [PubMed] [Google Scholar]
- Pisters P. W., Pachter H. L. Autologous splenic transplantation for splenic trauma. Ann Surg. 1994 Mar;219(3):225–235. doi: 10.1097/00000658-199403000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. M., James P. D. Ectopic splenic tissue failed to prevent fatal pneumococcal septicaemia after splenectomy for trauma. Lancet. 1980 Mar 15;1(8168 Pt 1):565–566. doi: 10.1016/s0140-6736(80)91056-9. [DOI] [PubMed] [Google Scholar]
- Rudowski W. J. Accessory spleens: clinical significance with particular reference to the recurrence of idiopathic thrombocytopenic purpura. World J Surg. 1985 Jun;9(3):422–430. doi: 10.1007/BF01655277. [DOI] [PubMed] [Google Scholar]
- Stovall T. G., Ling F. W. Splenosis: report of a case and review of the literature. Obstet Gynecol Surv. 1988 Feb;43(2):69–72. [PubMed] [Google Scholar]
- Timens W., Boes A., Rozeboom-Uiterwijk T., Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989 Nov 15;143(10):3200–3206. [PubMed] [Google Scholar]
- Timens W., Leemans R. Splenic autotransplantation and the immune system. Adequate testing required for evaluation of effect. Ann Surg. 1992 Mar;215(3):256–260. doi: 10.1097/00000658-199203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcek F. T., Kugaczewski J. T., Jongco B., Shaftan G. W., Rao P. S., Schiffman G., Kottmeier P. K. Function of the replanted spleen in dogs. J Trauma. 1982 Jun;22(6):502–506. doi: 10.1097/00005373-198206000-00011. [DOI] [PubMed] [Google Scholar]
- Weits J., de Gast G. C., The T. H., Esselink M. T., Deelder A. M., Petrovic M., Mandema E. Class-specific antibody titres (ELISA) against the primary immunogen Helix pomatia haemocyanin (HPH) in man. Clin Exp Immunol. 1978 Jun;32(3):443–450. [PMC free article] [PubMed] [Google Scholar]
- de Gast G. C., The T. H., Snijder J. A. The human immune response to alpha-haemocyanin of Helix pomatia. Acta Med Scand. 1973 Oct;194(4):303–309. doi: 10.1111/j.0954-6820.1973.tb19450.x. [DOI] [PubMed] [Google Scholar]


