Abstract
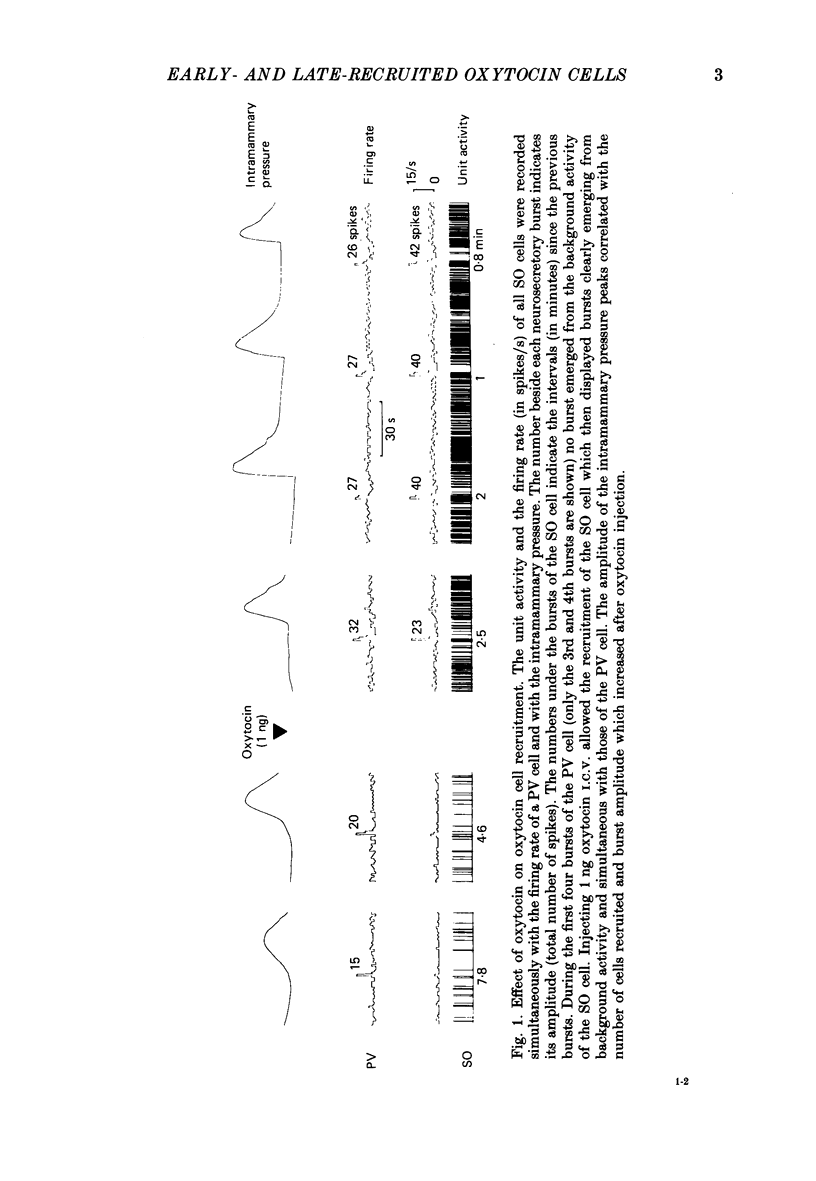
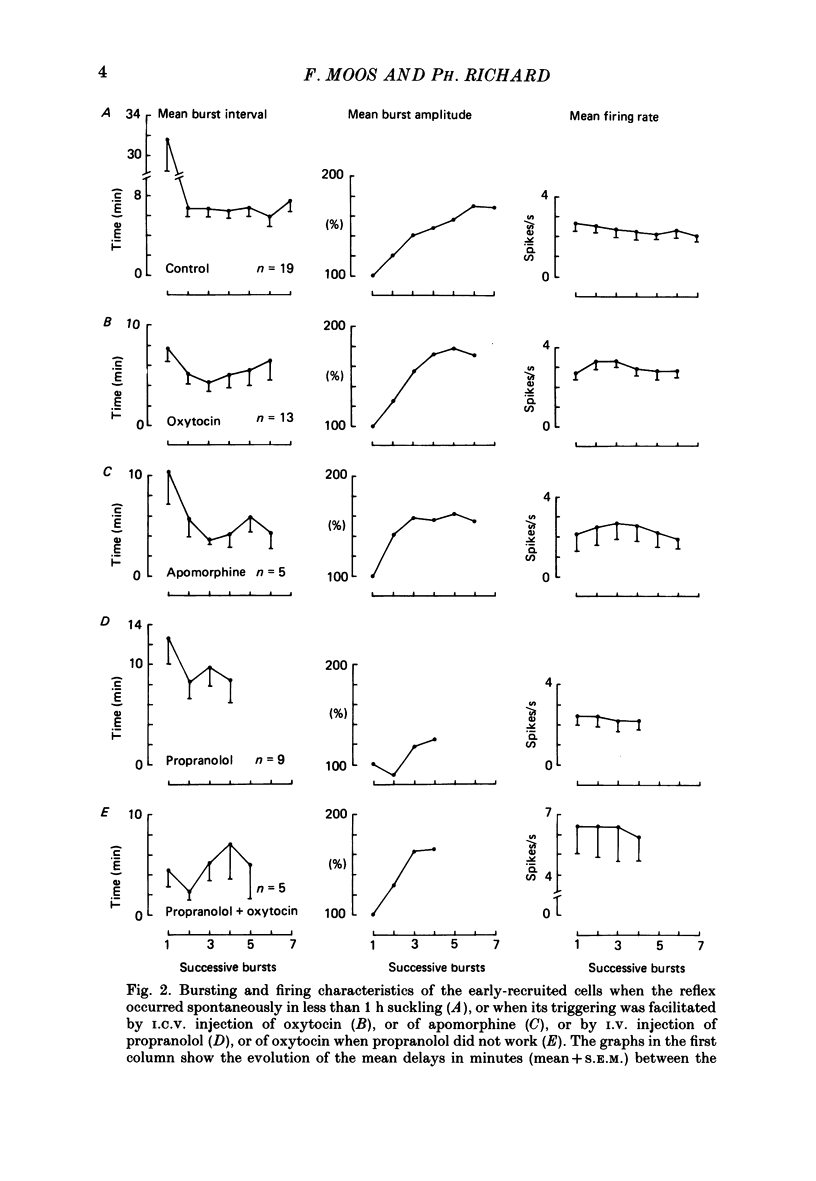
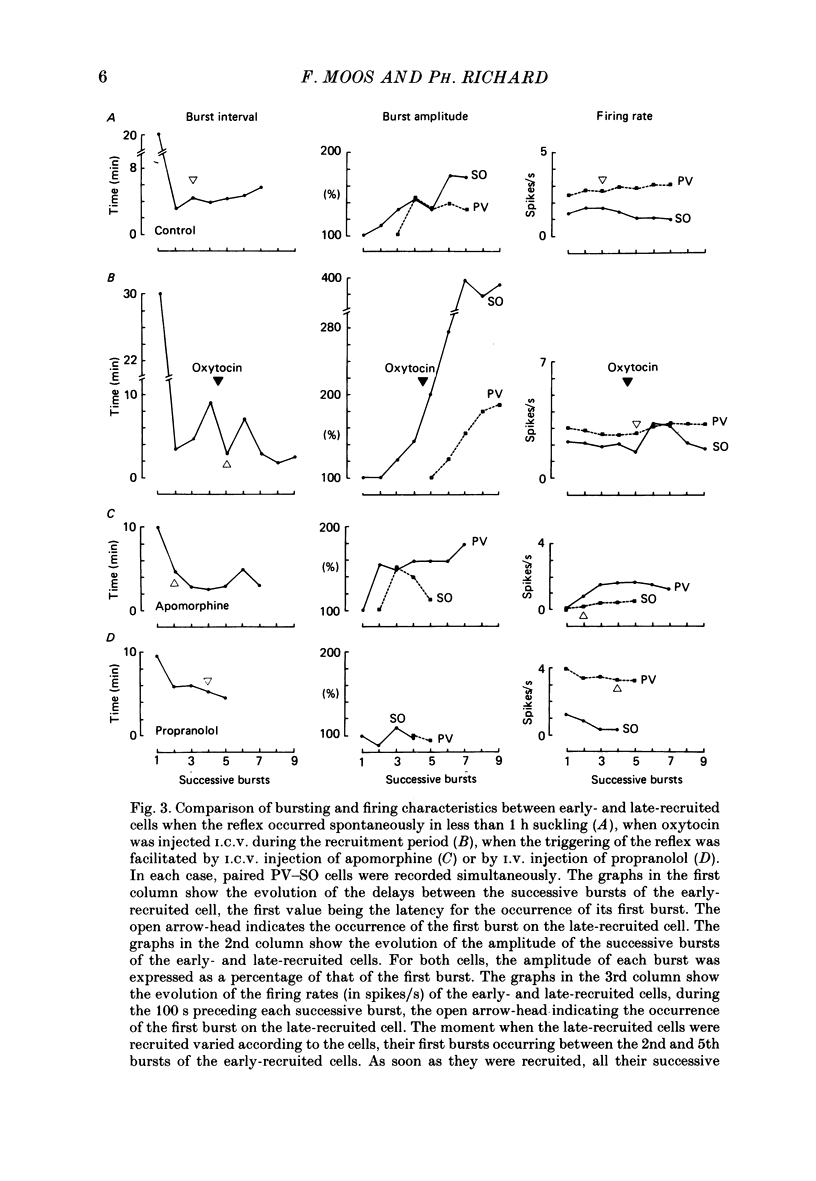
1. Paired or single recordings of paraventricular and/or supraoptic oxytocin cells at the beginning of suckling in urethane-anaesthetized rats enabled us to study cell recruitment and compare the characteristics of the early- and late-recruited cells. This was done under different experimental conditions, i.e. when the reflex was triggered in less than 1 h suckling (control), and when its triggering was facilitated either by the intraventricular (i.c.v.) injection of oxytocin, of apomorphine (a dopamine agonist) or by the intravenous (i.v.) injection of propranolol (a beta-adrenoceptor antagonist) into suckled rats with no milk ejection. 2. Under control conditions, the amplitude (total number of spikes) of the successive bursts of the early-recruited cells progressively increased, generally reaching maximum by the 6th burst. This increase was more rapid and greater after oxytocin than under control conditions or after apomorphine injection, and was delayed and reduced after propranolol. The burst frequency was higher after oxytocin and apomorphine injections than under control conditions and very low after propranolol. 3. Late-recruited cells were observed under all experimental conditions, except after oxytocin injection, since all cells displayed bursts right away. Moreover, when injected during the recruitment period of a control reflex, oxytocin greatly speeded up the recruitment of the late-recruited cells. These cells generally displayed smaller amplitude bursts than the early-recruited cells. Moreover, the increase in burst amplitude was less marked for the late- than for the early-recruited cells and often was not sustained. 4. Neither the likelihood of recruitment of an oxytocin cell nor its burst amplitude could be correlated with background activity level and there was no clear relationship between the recruitment period or the bursting characteristics on one hand and the background activity on the other. 5. In conclusion, the differences between the early- and late-recruited cells in recruitment time and in burst amplitude reflected differences in cell excitability which may depend mainly on the presence of oxytocin in the magnocellular nuclei.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin V., Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986 Aug;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Non-synaptic depolarizing potentials in rat supraoptic neurones recorded in vitro. J Physiol. 1986 Jul;376:493–505. doi: 10.1113/jphysiol.1986.sp016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. A., Randle J. C., Renaud L. P. Opposing alpha- and beta-adrenergic mechanisms mediate dose-dependent actions of noradrenaline on supraoptic vasopressin neurones in vivo. Brain Res. 1985 Dec 9;358(1-2):171–179. doi: 10.1016/0006-8993(85)90961-8. [DOI] [PubMed] [Google Scholar]
- Day T. A., Renaud L. P. Electrophysiological evidence that noradrenergic afferents selectively facilitate the activity of supraoptic vasopressin neurons. Brain Res. 1984 Jun 15;303(2):233–240. doi: 10.1016/0006-8993(84)91209-5. [DOI] [PubMed] [Google Scholar]
- Di Scala-Guenot D., Strosser M. T., Richard P. Electrical stimulations of perifused magnocellular nuclei in vitro elicit Ca2+-dependent, tetrodotoxin-insensitive release of oxytocin and vasopressin. Neurosci Lett. 1987 May 6;76(2):209–214. doi: 10.1016/0304-3940(87)90717-8. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Negoro H., Fukuoka T., Higuchi T., Uchide K. Effect of microelectrophoretically applied acetylcholine, noradrenaline, dopamine and serotonin on the discharge of paraventricular oxytocinergic neurones in the rat. Endocrinol Jpn. 1985 Feb;32(1):127–133. doi: 10.1507/endocrj1954.32.127. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hill A., Wakerley J. B. The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol. 1973 Jun;57(3):459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol. 1975 Sep;250(2):443–461. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T. Excitation by dopamine of putative oxytocinergic neurones in the rat supraoptic nucleus in vitro: evidence for two classes of continuously firing neurones. Brain Res. 1983 May 9;267(1):113–121. doi: 10.1016/0006-8993(83)91044-2. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Hatton G. I., Ho Y. W., Chapman C., Robinson I. C. Central release of oxytocin, vasopressin and neurophysin by magnocellular neurone depolarization: evidence in slices of guinea pig and rat hypothalamus. Neuroendocrinology. 1986;42(4):311–322. doi: 10.1159/000124457. [DOI] [PubMed] [Google Scholar]
- Meyer C., Freund-Mercier M. J., Richard P. Facilitatory effect of oxytocin on oxytocin cell background activity in the rat is suckling-dependent. Neurosci Lett. 1987 Mar 20;75(1):80–84. doi: 10.1016/0304-3940(87)90079-6. [DOI] [PubMed] [Google Scholar]
- Moos F., Freund-Mercier M. J., Guerné Y., Guerné J. M., Stoeckel M. E., Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984 Jul;102(1):63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Excitatory effect of dopamine on oxytocin and vasopressin reflex releases in the rat. Brain Res. 1982 Jun 10;241(2):249–260. doi: 10.1016/0006-8993(82)91061-7. [DOI] [PubMed] [Google Scholar]
- Nakada H., Nakai Y. Electron microscopic examination of the catecholaminergic innervation of neurophysin- or vasopressin-containing neurons in the rat hypothalamus. Brain Res. 1985 Dec 30;361(1-2):247–257. doi: 10.1016/0006-8993(85)91296-x. [DOI] [PubMed] [Google Scholar]
- Ogata N., Matsuo T. The effects of catecholamines on electrical activity of neurons in the guinea pig supraoptic nucleus in vitro. Brain Res. 1986 Oct 15;385(1):122–135. doi: 10.1016/0006-8993(86)91553-2. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Dyer R. G. Reproducible increases in intramammary pressure after spinal cord stimulation in lactating rats. Exp Brain Res. 1984;55(2):313–316. doi: 10.1007/BF00237281. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Clarke G., Dreifuss J. J., Lincoln D. W. The role of central adrenergic receptors in the reflex release of oxytocin. Brain Res. 1978 Feb 17;142(1):69–84. doi: 10.1016/0006-8993(78)90177-4. [DOI] [PubMed] [Google Scholar]
- Zhu P. C., Thureson-Klein A., Klein R. L. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience. 1986 Sep;19(1):43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]


