Abstract
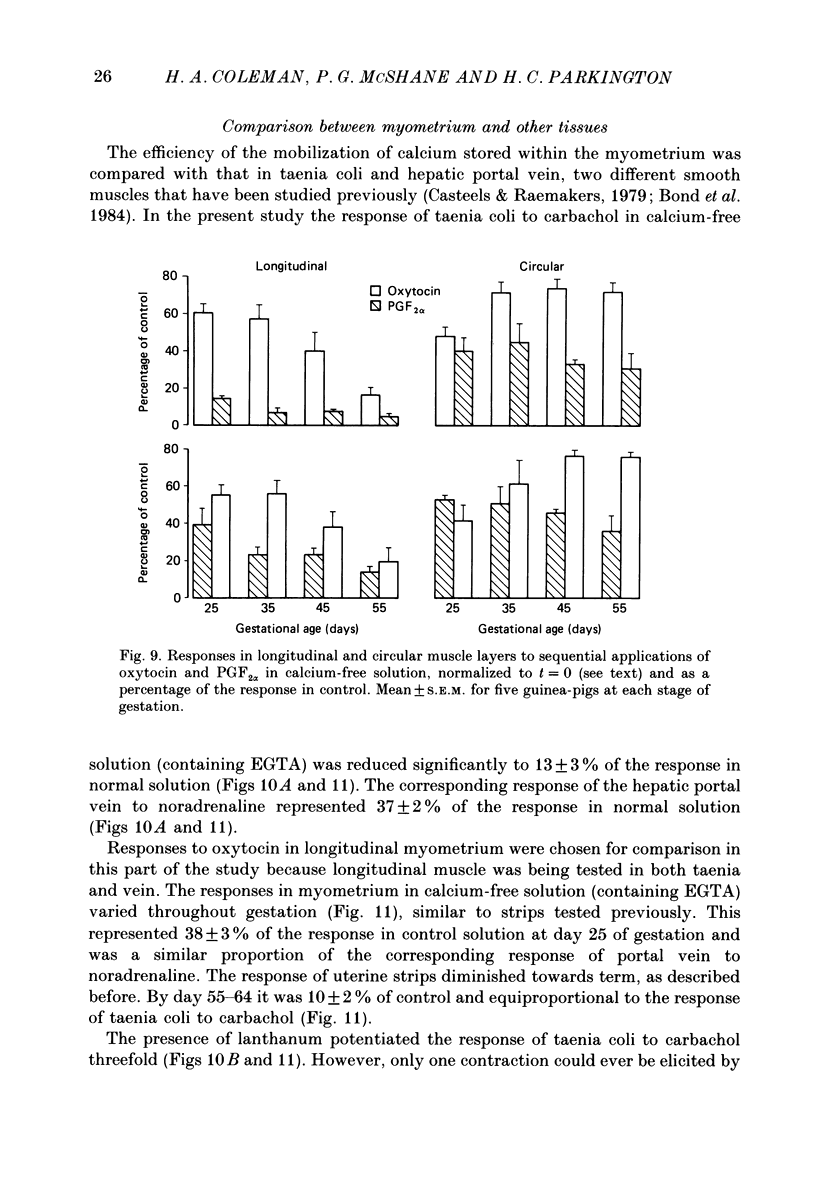
1. The ability of oxytocin and prostaglandin F2 alpha (PGF) to induce contraction in guinea-pig myometrium in calcium-free solution was studied in an attempt to assess the extent to which intracellular calcium stores could be released by these two agonists. Both longitudinal and circular muscle layers were studied separately and the effects of gestational age were also examined. 2. In longitudinal strips, the responses to oxytocin and PGF in the absence of external calcium decreased progressively throughout gestation. Responses of circular strips to both agonists were unchanged throughout pregnancy, until day 64, when no response to PGF could be elicited. 3. Pre-treatment with high potassium (and normal calcium) increased the responses to the agonists in calcium-free medium while pre-treatment with beta-adrenoceptor agonists had no effect on responses to oxytocin or PGF. 4. Responses to both agonists decreased with time in calcium-free solution suggesting a loss of calcium from stores with a half-time of 3 min. The rate of the decline in the responses was the same in both muscle layers and did not change with gestational age. 5. In the presence of lanthanum contractions evoked by oxytocin, but not PGF, were augmented 2-3-fold. This potentiation of the response to oxytocin occurred in both muscle layers and throughout gestation. 6. Each agonist evoked only one response in calcium-free solution containing EGTA. The response to PGF in longitudinal strips following a challenge with oxytocin was reduced, compared with the response to PGF when applied first while the response to oxytocin in these strips was unchanged following exposure to PGF. In circular strips neither oxytocin- nor PGF-induced contractions were altered following prior exposure to the other agonist. 7. It is concluded that oxytocin and PGF operate via two distinct mechanisms to release intracellularly stored calcium in both longitudinal and circular components of the guinea-pig myometrium and a hypothesis to explain the results is presented.
Full text
PDF


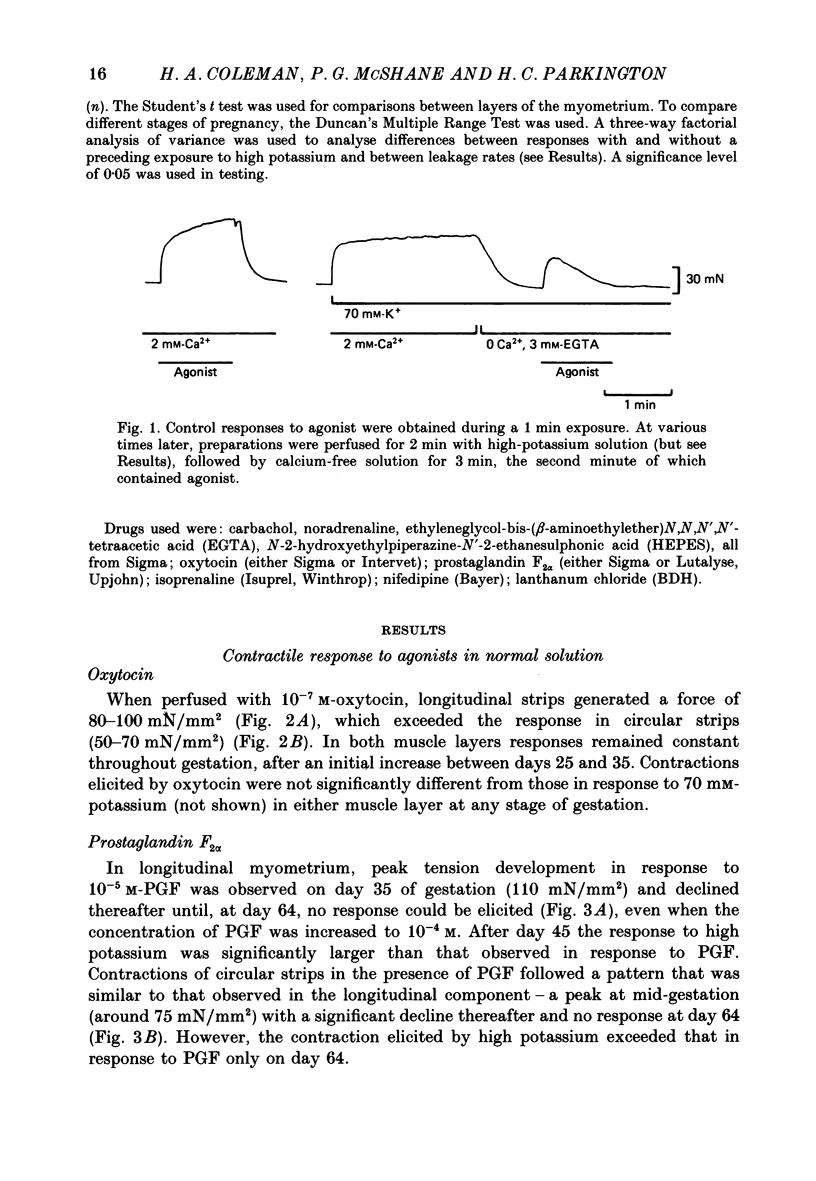
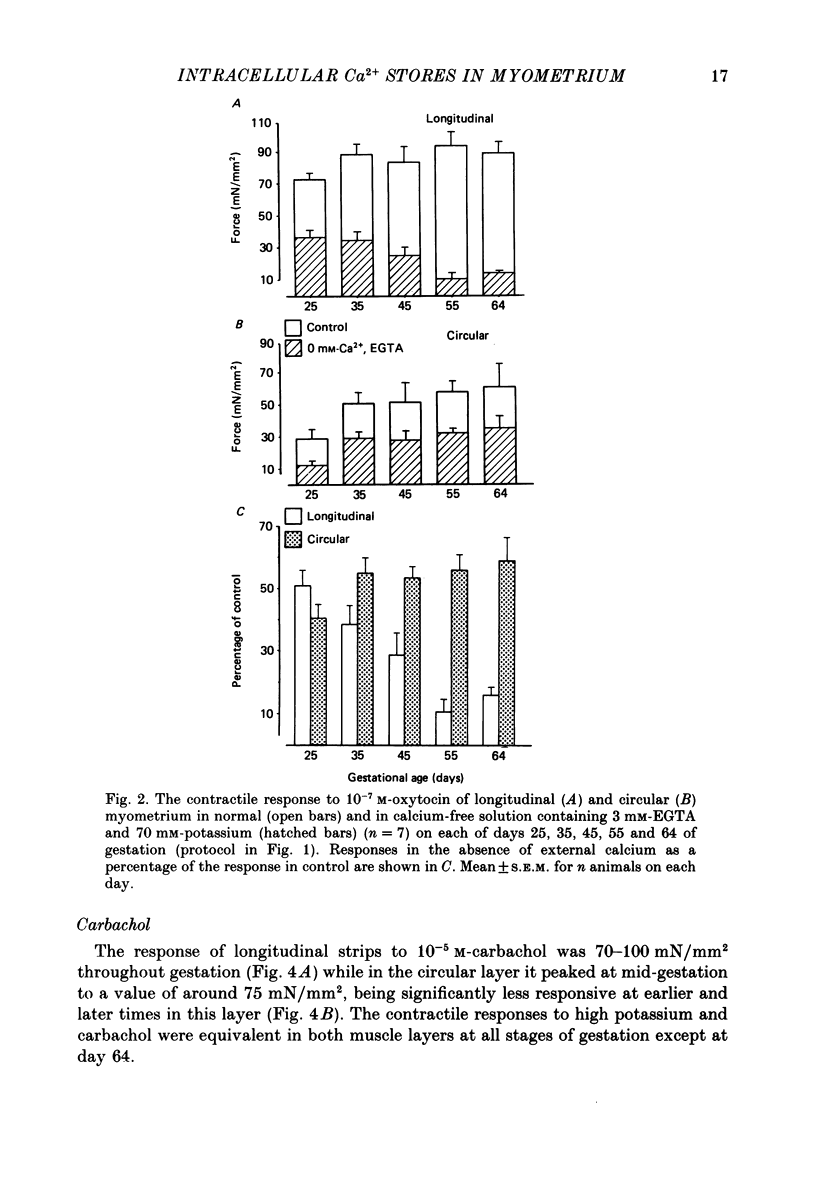
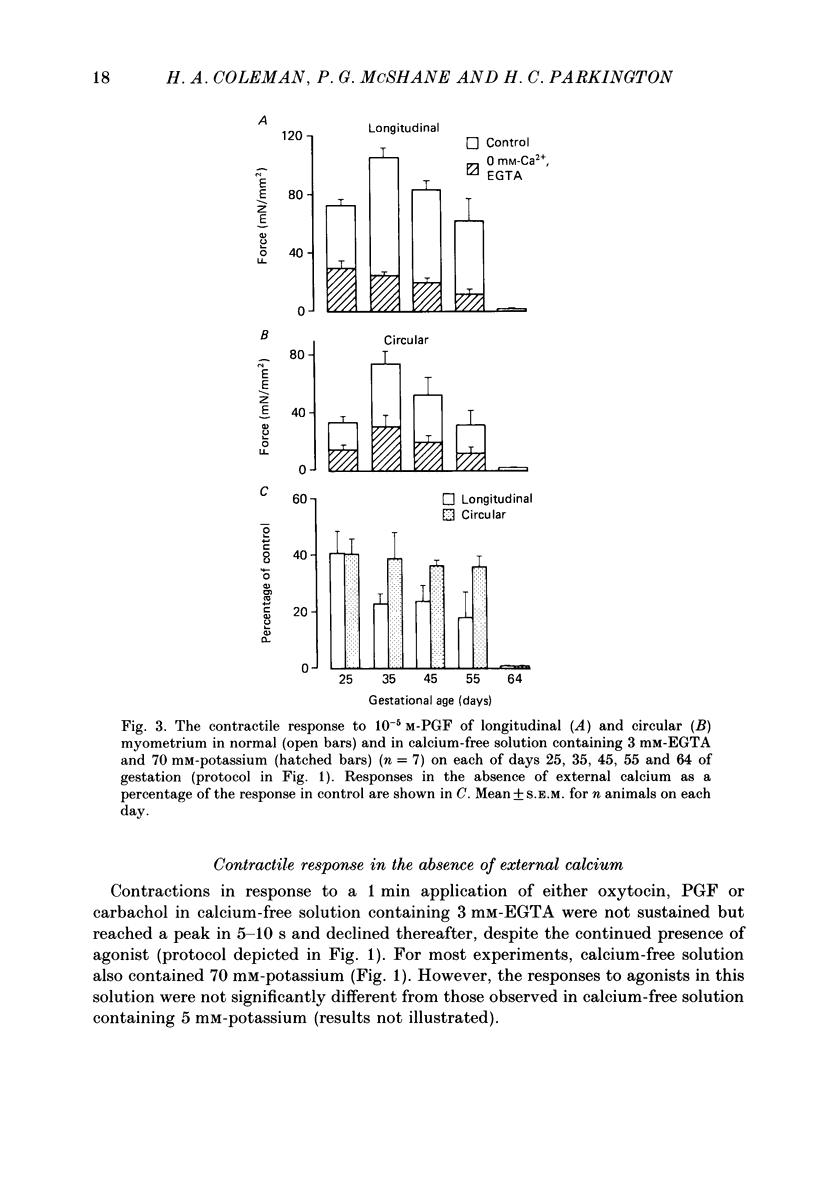
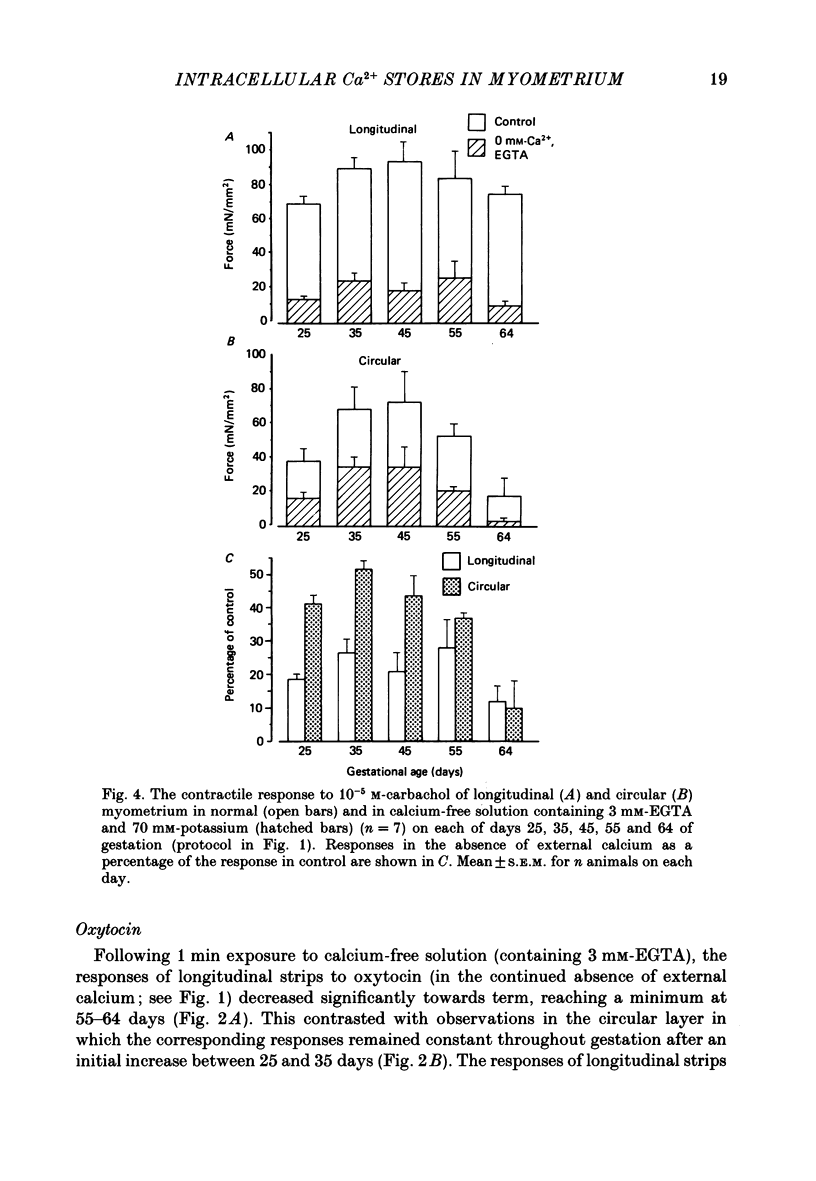






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashoori F., Tomita T. Mechanical response to noradrenaline in calcium-free solution in the rat vas deferens. J Physiol. 1983 May;338:165–178. doi: 10.1113/jphysiol.1983.sp014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
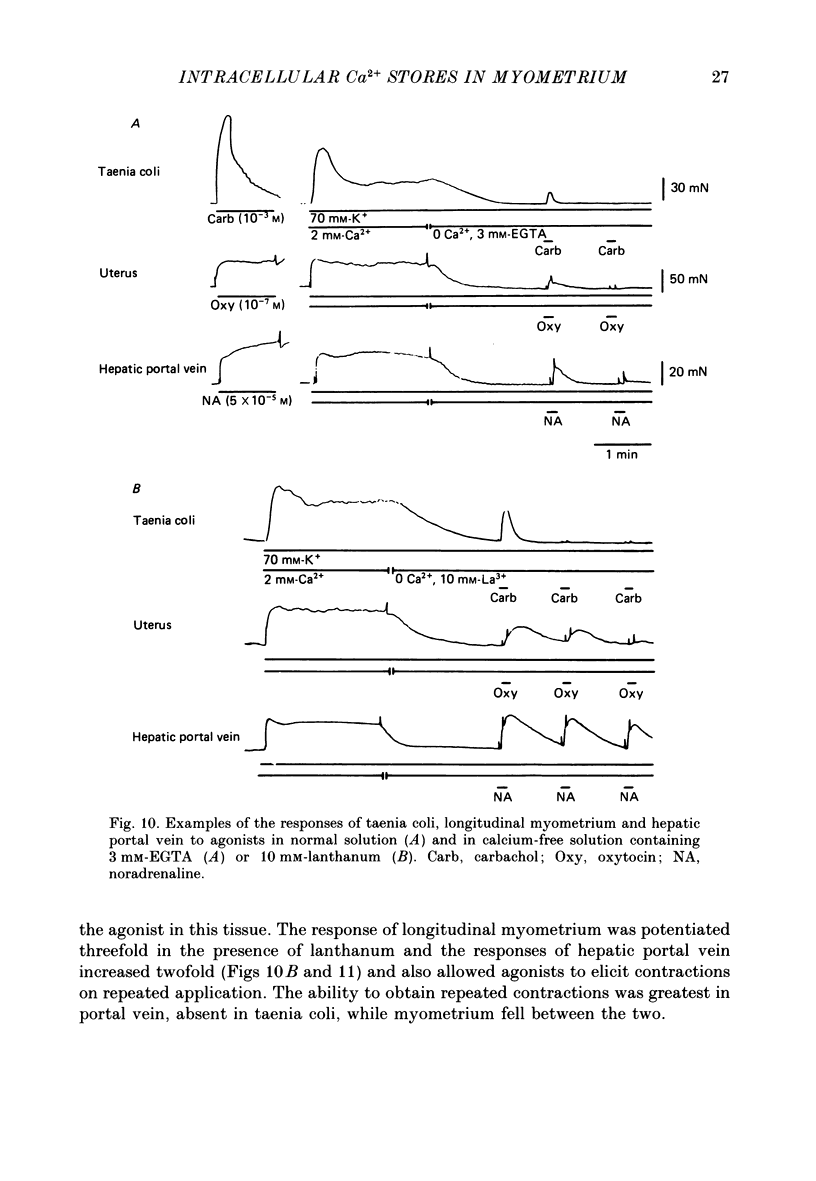
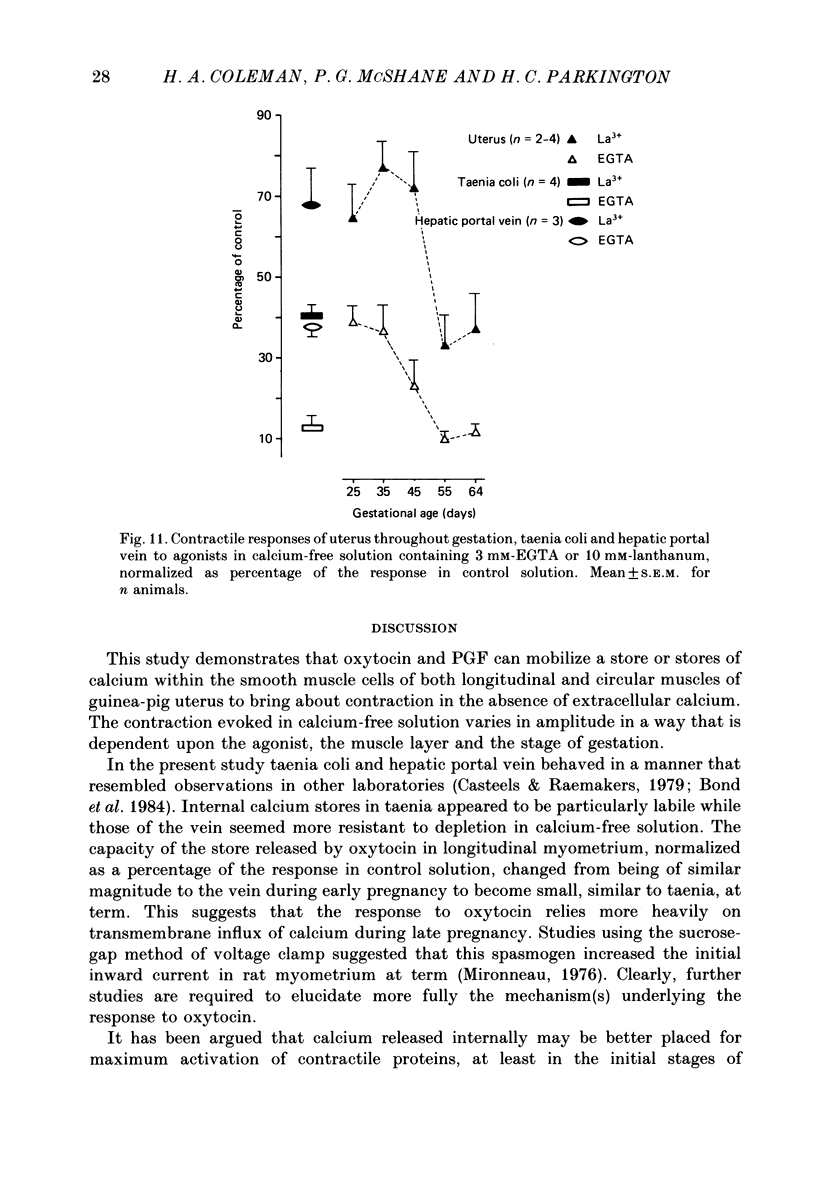
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsten M. E., Miller J. D. Ca2+ release by inositol trisphosphate from Ca2+-transporting microsomes derived from uterine sarcoplasmic reticulum. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1027–1031. doi: 10.1016/0006-291x(85)91718-8. [DOI] [PubMed] [Google Scholar]
- Carsten M. E. Prostaglandins and oxytocin: their effects on uterine smooth muscle. Prostaglandins. 1974 Jan 10;5(1):33–40. doi: 10.1016/s0090-6980(74)80128-0. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley W. A., Parkington H. C. Relaxin inhibits the plateau component of the action potential in the circular myometrium of the rat. J Physiol. 1984 Aug;353:51–65. doi: 10.1113/jphysiol.1984.sp015321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E. H., Marshall J. M. Effects of catecholamines on circular and longitudinal uterine muscle of the rat. Eur J Pharmacol. 1981 Dec 3;76(2-3):157–165. doi: 10.1016/0014-2999(81)90497-0. [DOI] [PubMed] [Google Scholar]
- Deth R. C. Effect of lanthanum and reduced temperature on 45Ca efflux from rabbit aorta. Am J Physiol. 1978 May;234(5):C139–C145. doi: 10.1152/ajpcell.1978.234.5.C139. [DOI] [PubMed] [Google Scholar]
- Deth R. C., Lynch C. J. Mobilization of a common source of smooth muscle Ca2+ by norepinephrine and methylxanthines. Am J Physiol. 1981 May;240(5):C239–C247. doi: 10.1152/ajpcell.1981.240.5.C239. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Rojas H. R., Beaugé L. Vanadate inhibits uncoupled Ca efflux but not Na--Ca exchange in squid axons. Nature. 1979 Sep 20;281(5728):229–230. doi: 10.1038/281228a0. [DOI] [PubMed] [Google Scholar]
- EDMAN K. A., SCHILD H. O. The need for calcium in the contractile responses induced by acetylcholine and potassium in the rat uterus. J Physiol. 1962 May;161:424–441. doi: 10.1113/jphysiol.1962.sp006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O. Mechanism of contraction of smooth muscle by drugs. Nature. 1957 Aug 17;180(4581):341–342. doi: 10.1038/180341c0. [DOI] [PubMed] [Google Scholar]
- Godfraind T. Calcium exchange in vascular smooth muscle, action of noradrenaline and lanthanum. J Physiol. 1976 Aug;260(1):21–35. doi: 10.1113/jphysiol.1976.sp011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson B. J., Daniel E. E. Studies concerning the source of calcium for contraction of rat myometrium. Can J Physiol Pharmacol. 1973 Dec;51(12):914–932. doi: 10.1139/y73-140. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Ueno H., Kuriyama H. Calcium-induced calcium release mechanism in vascular smooth muscles--assessments based on contractions evoked in intact and saponin-treated skinned muscles. Experientia. 1985 Aug 15;41(8):989–996. doi: 10.1007/BF01952119. [DOI] [PubMed] [Google Scholar]
- Lalanne C., Mironneau C., Mironneau J., Savineau J. P. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+-free medium. Br J Pharmacol. 1984 Feb;81(2):317–326. doi: 10.1111/j.1476-5381.1984.tb10081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Carbachol and oxytocin stimulate the generation of inositol phosphates in the guinea pig myometrium. FEBS Lett. 1986 May 26;201(1):9–14. doi: 10.1016/0014-5793(86)80561-0. [DOI] [PubMed] [Google Scholar]
- Mironneau C., Mironneau J., Savineau J. P. Maintained contractions of rat uterine smooth muscle incubated in a Ca2+-free solution. Br J Pharmacol. 1984 Jul;82(3):735–743. doi: 10.1111/j.1476-5381.1984.tb10813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J. Effects of oxytocin on ionic currents underlying rhythmic activity and contraction in uterine smooth muscle. Pflugers Arch. 1976 May 12;363(2):113–118. doi: 10.1007/BF01062278. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Osa T., Katase T. Physiological comparison of the longitudinal and circular muscles of the pregnant rat uterus. Jpn J Physiol. 1975;25(2):153–164. doi: 10.2170/jjphysiol.25.153. [DOI] [PubMed] [Google Scholar]
- Parkington H. C. Some properties of the circular myometrium of the sheep throughout pregnancy and during labour. J Physiol. 1985 Feb;359:1–15. doi: 10.1113/jphysiol.1985.sp015571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Higuchi K., Yamaguchi T., Uchida M. Oxytocin-induced Ca-free contraction of rat uterine smooth muscle: effects of preincubation with EGTA and drugs. Gen Pharmacol. 1982;13(5):393–400. doi: 10.1016/0306-3623(82)90104-5. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Wuytack F., Casteels R., Martinelli B., Campailla E., Ferrari G. Stimulation of 45Ca efflux from smooth muscle cells by metabolic inhibition and high K depolarization. Pflugers Arch. 1975 Sep 9;359(3):183–196. doi: 10.1007/BF00587378. [DOI] [PubMed] [Google Scholar]
- Villar A., D'Ocon M. P., Anselmi E. Calcium requirement of uterine contraction induced by PGE1: importance of intracellular calcium stores. Prostaglandins. 1985 Sep;30(3):491–496. doi: 10.1016/0090-6980(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]


