Abstract
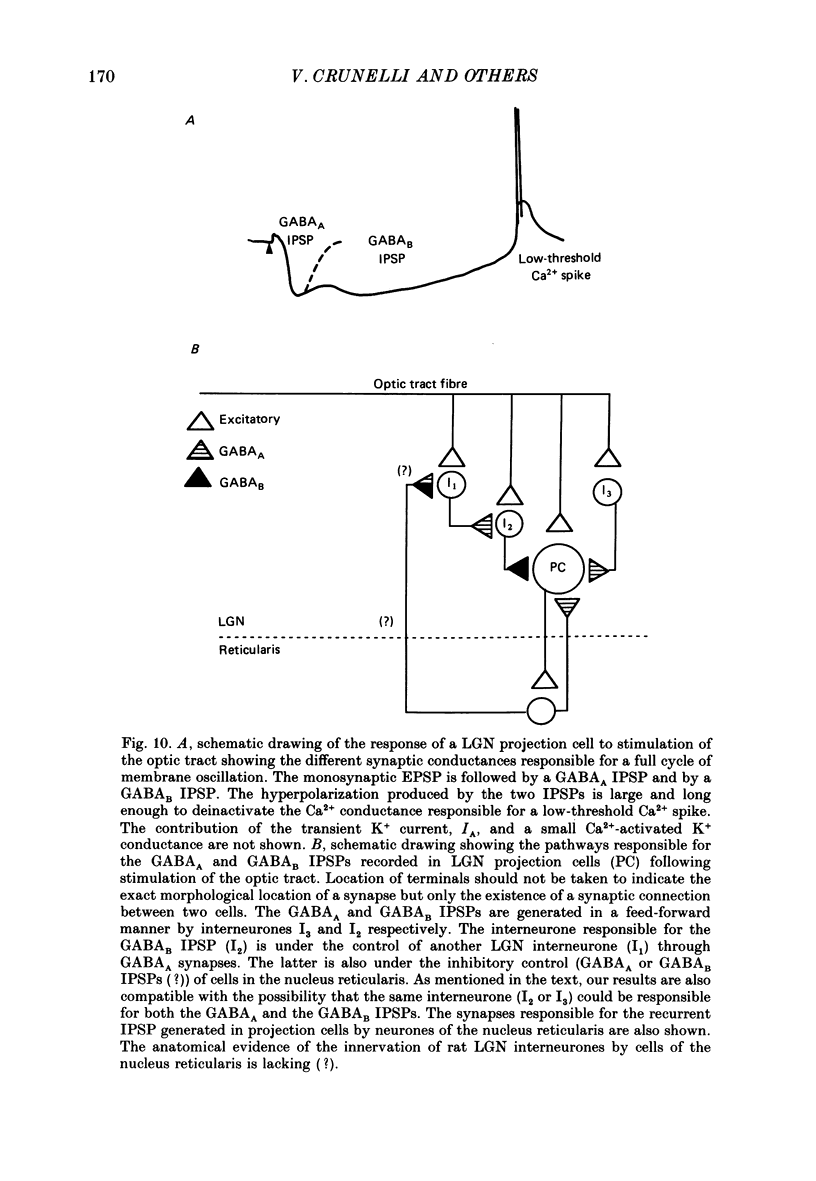
1. Hyperpolarizing potentials evoked by electrical stimulation of the optic tract were studied in projection cells of the rat dorsal lateral geniculate nucleus (LGN) in vitro. In the same cells the effects of gamma-amino butyric acid (GABA), baclofen and acetylcholine (ACh) were also investigated. 2. In the majority of cells a short- (SHP) (34 ms) and a long-lasting (LHP) (240 ms) hyperpolarizing potential could be recorded in the presence and in the absence of a preceding EPSP. They were blocked by tetrodotoxin (1 microM) and were more sensitive than the monosynaptic EPSP to a low-Ca2+-high-Mg2+ solution. 3. The SHP was associated with a marked decrease (75%) in input resistance, was blocked by bicuculline (1-100 microM) and its reversal potential (-67 mV) was dependent on the extracellular Cl- concentration. 4. The LHP was associated with a smaller decrease (45%) in input resistance and its reversal potential (-76 mV) was dependent on the extracellular K+ concentration. It was increased by bicuculline (100% at 50 microM) and nipecotic acid (30% at 10 microM), blocked by Ba2+ (1 mM), and unaffected by eserine (1-10 microM), neostigmine (1-10 microM) or by recording with EGTA-filled electrodes. In the presence of bicuculline, a single LHP was able to evoke, as a rebound response, a low-threshold Ca2+ spike that was, however, not followed by another LHP (or any other long-lasting hyperpolarization). 5. Ionophoretic applications of GABA evoked in the same cell a Cl- -dependent hyperpolarization (reversal potential: -65 mV) and/or depolarization, both of which were associated with a marked decrease (91%) in input resistance and abolished by bicuculline. GABA was also able to evoke a bicuculline-insensitive, K+-dependent hyperpolarization that had a reversal potential of -75 mV and was associated with a smaller decrease (43%) in input resistance. 6. Baclofen, applied by ionophoresis, pressure ejection or in the perfusion medium (1-100 microM), produced a hyperpolarization that had a reversal potential of -79 mV and was associated with a decrease (45%) in input resistance. 7. In the majority of cells (thirty-seven out of forty) ACh evoked a slow depolarization and only in three cells a hyperpolarization which had a reversal potential of -80 mV.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


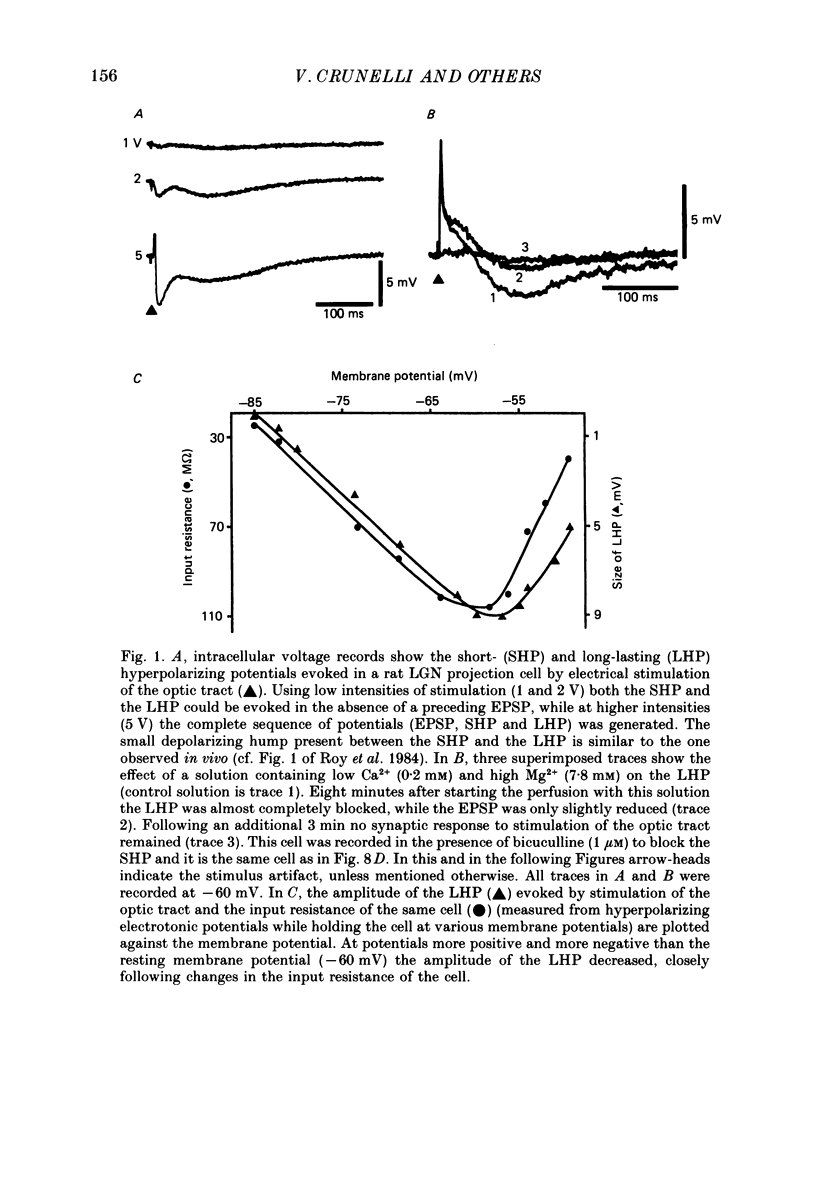

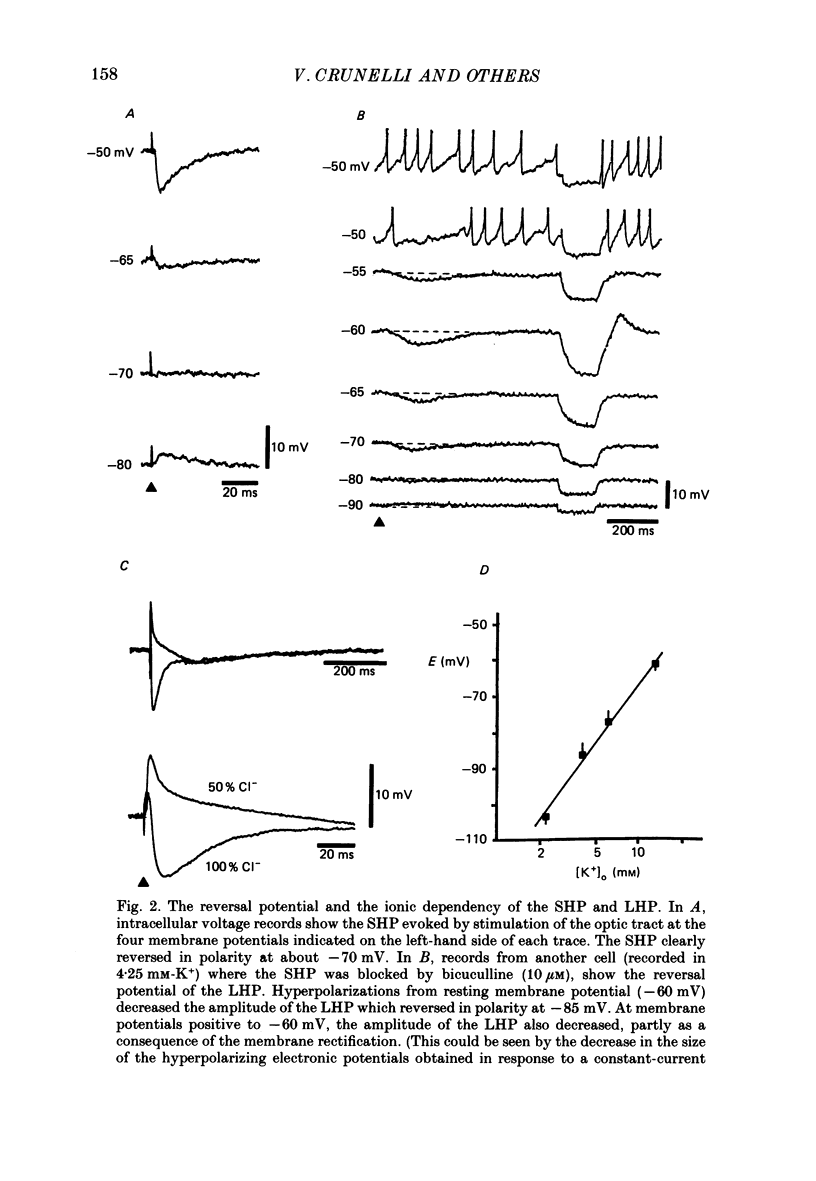

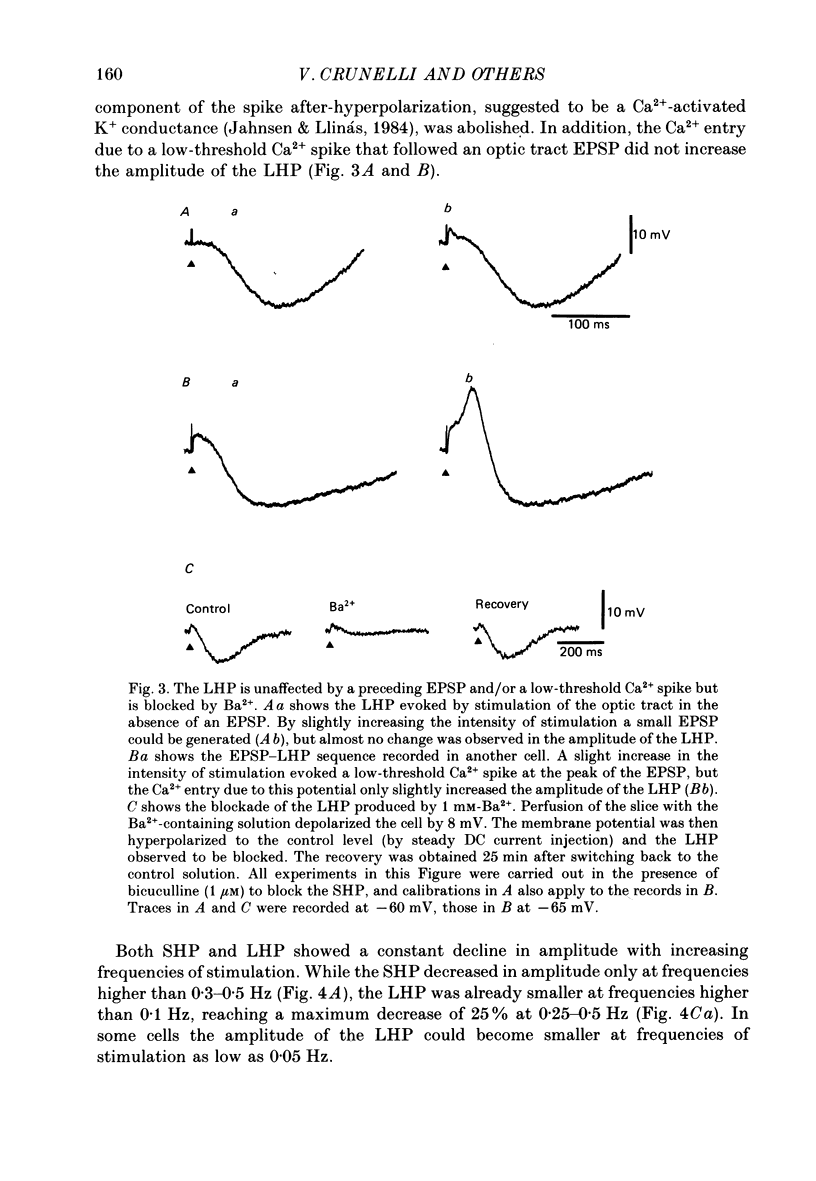
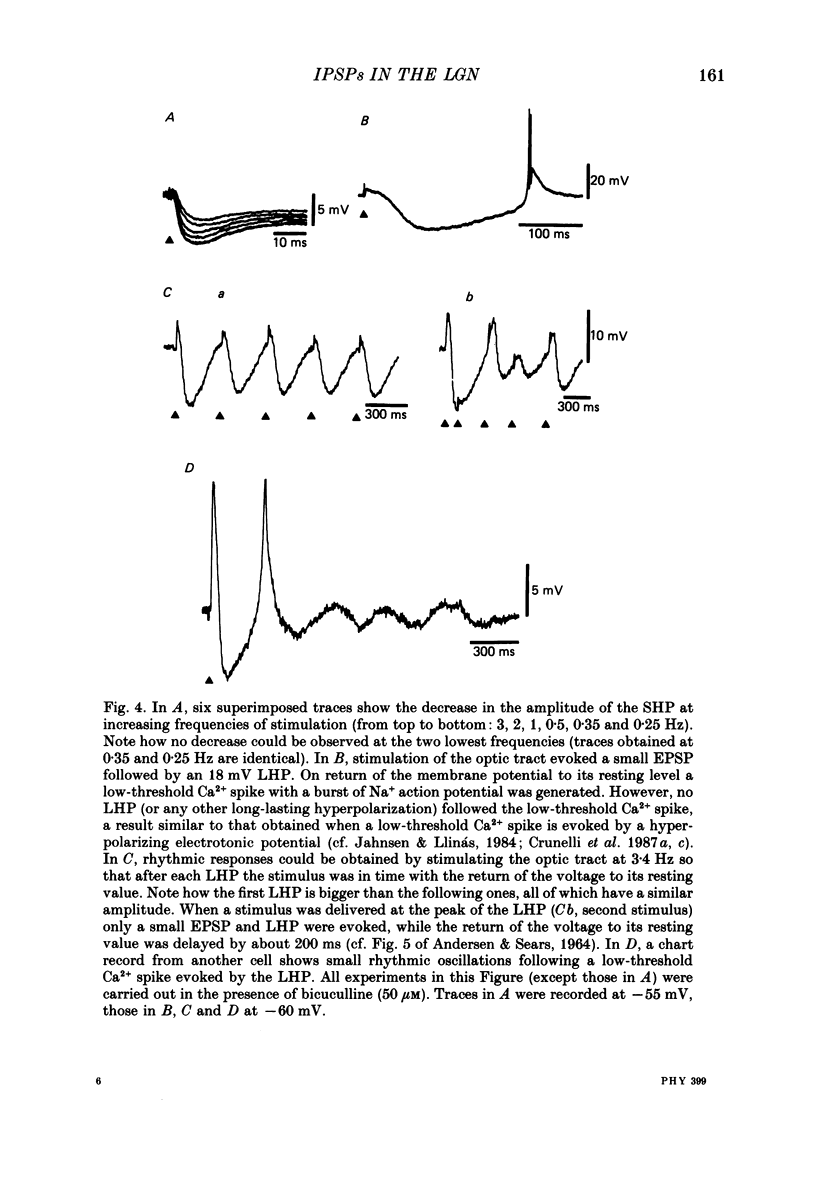
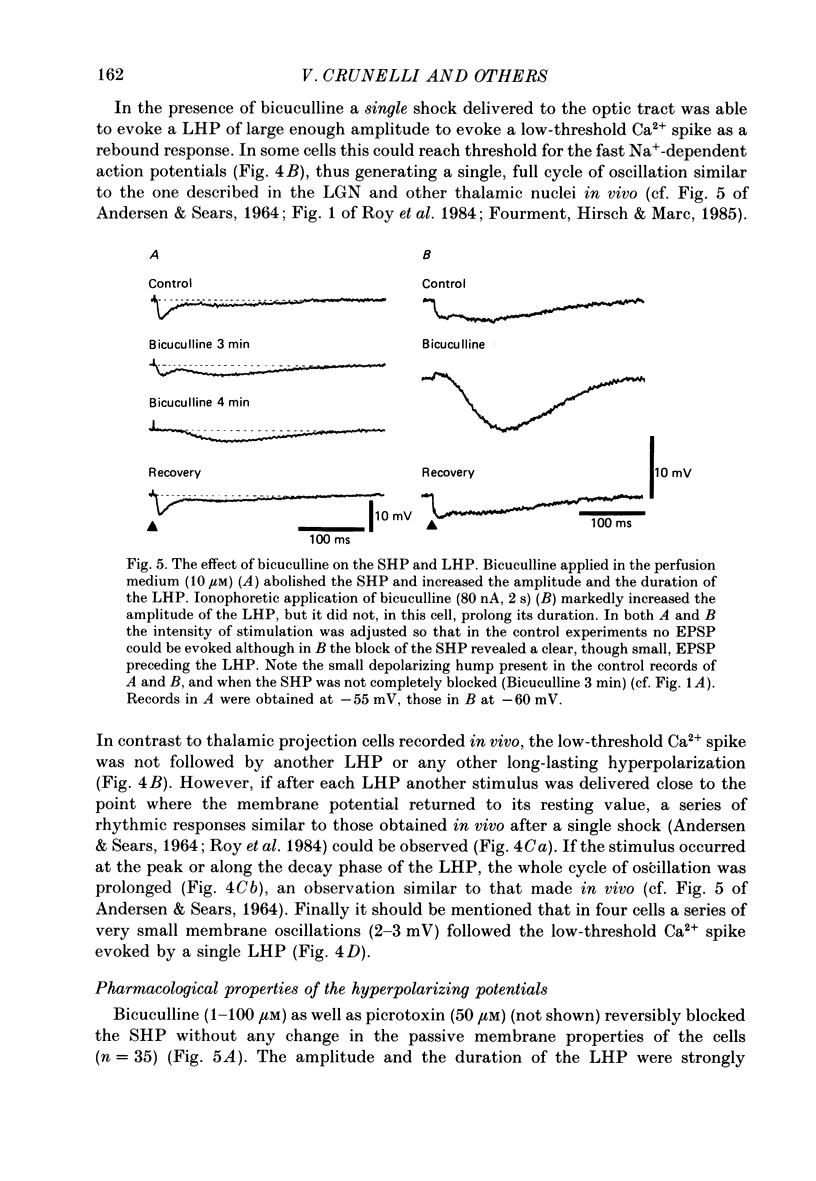
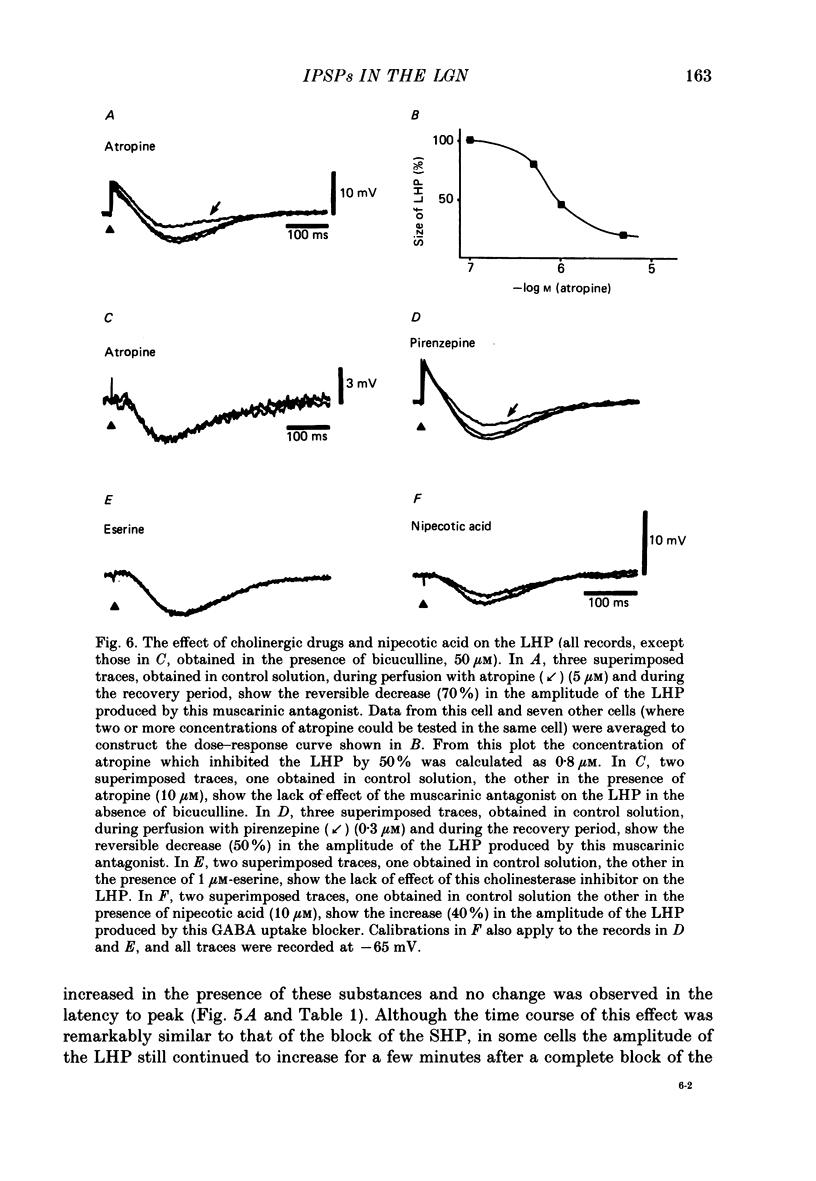

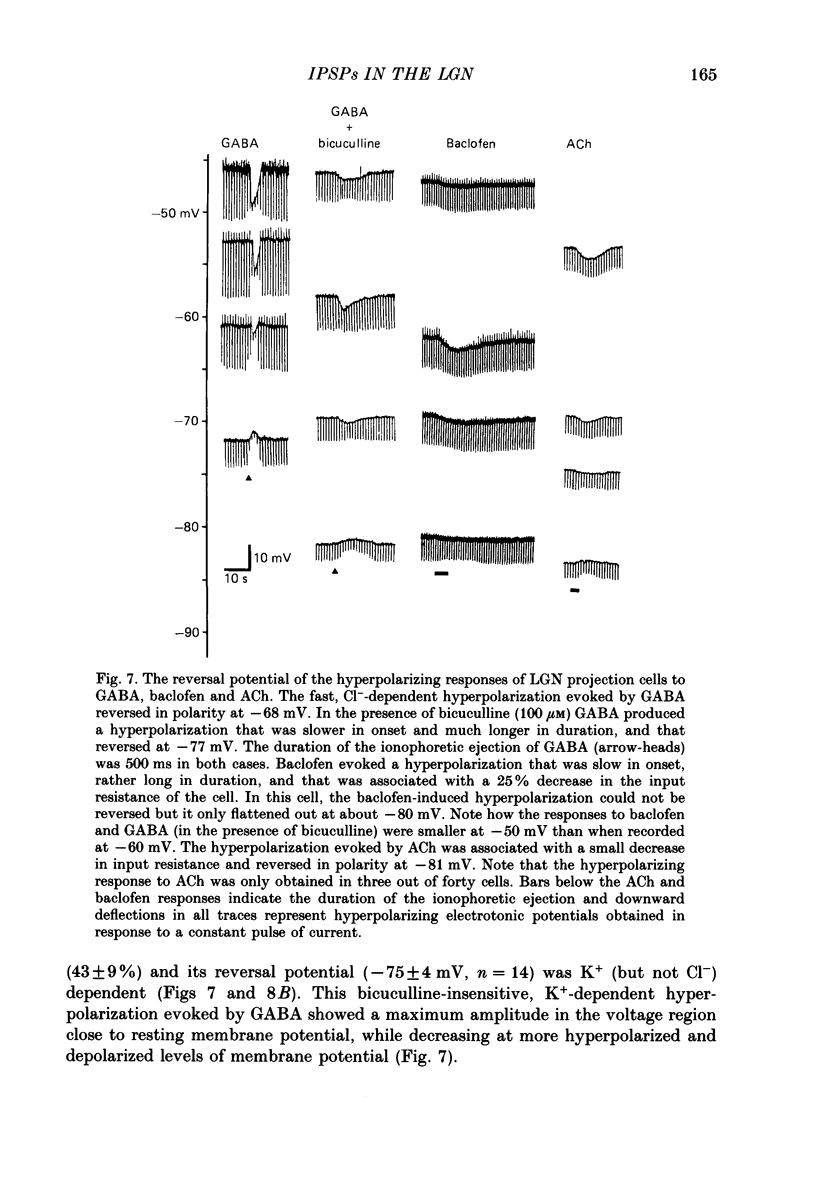
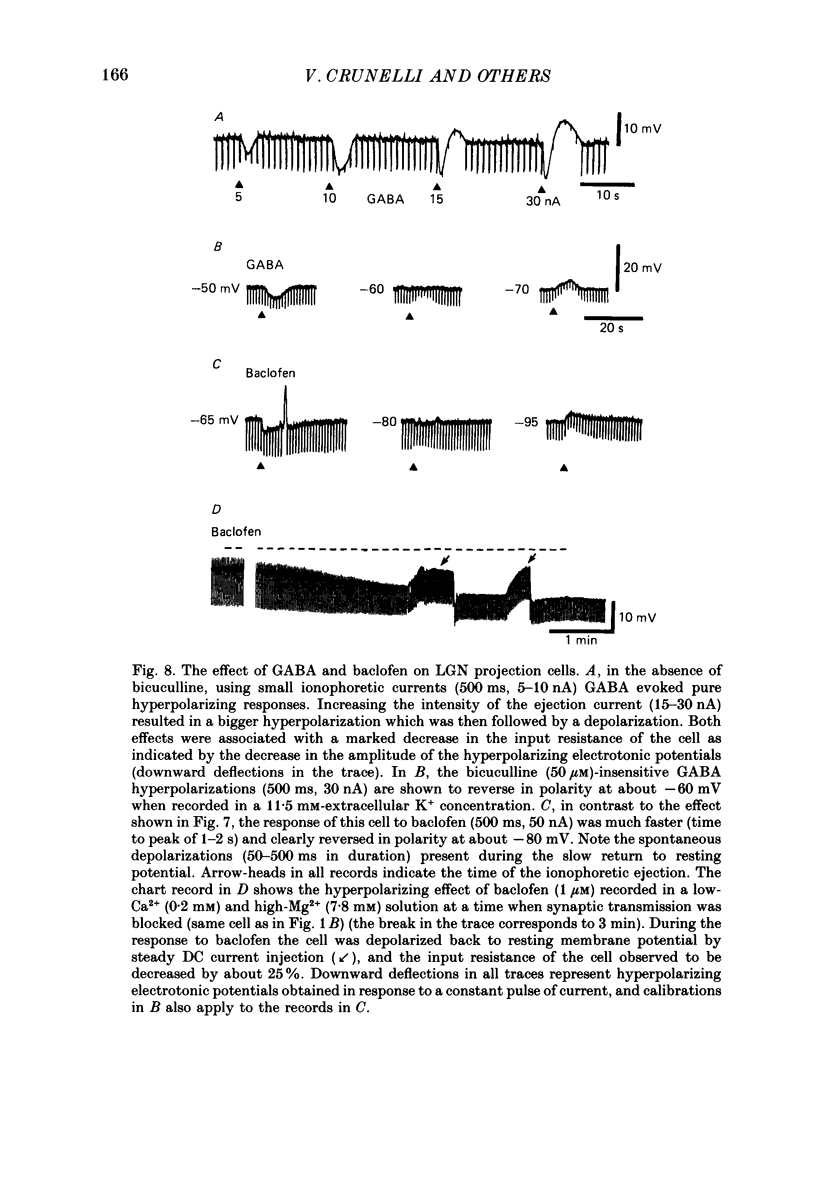








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
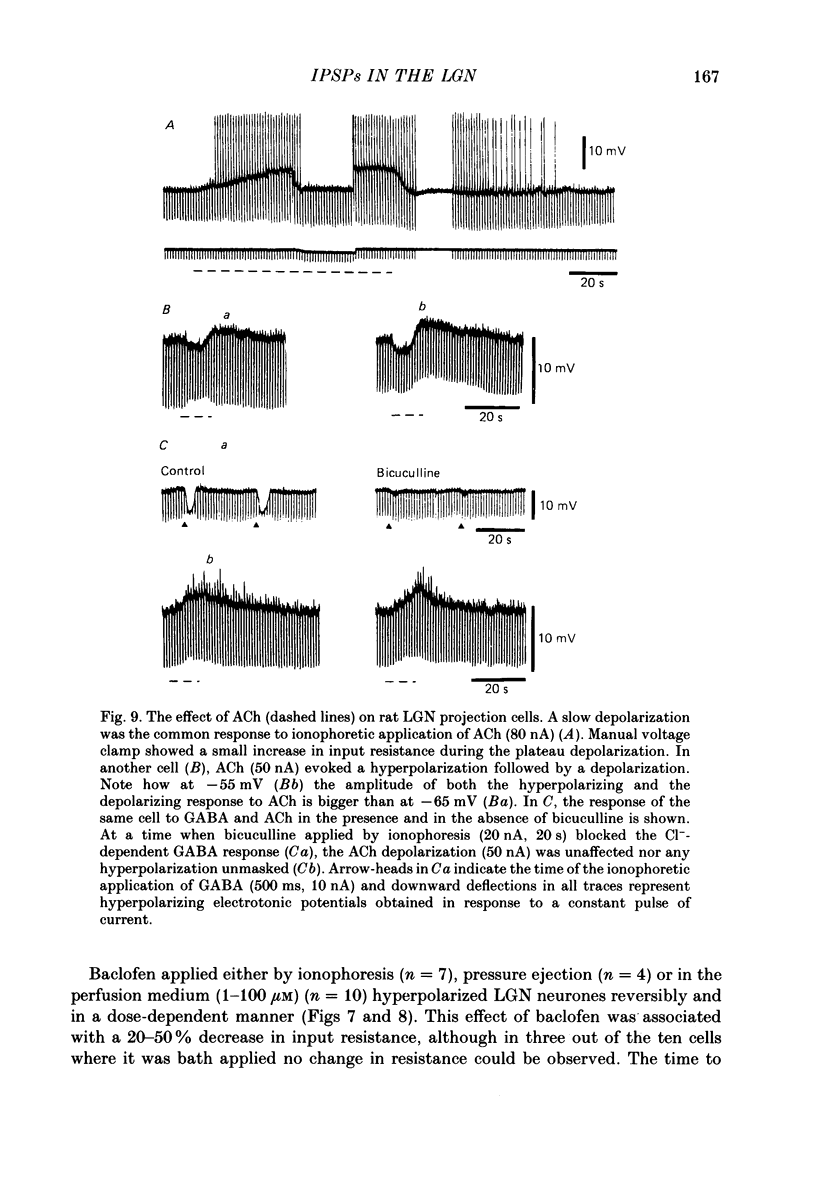
- Ahlsén G., Lindström S., Lo F. S. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Res. 1985;58(1):134–143. doi: 10.1007/BF00238961. [DOI] [PubMed] [Google Scholar]
- Alger B. E. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1984 Nov;52(5):892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. The reversal potential of excitatory amino acid action on granule cells of the rat dentate gyrus. J Physiol. 1984 Jun;351:327–342. doi: 10.1113/jphysiol.1984.sp015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. On the excitatory post-synaptic potential evoked by stimulation of the optic tract in the rat lateral geniculate nucleus. J Physiol. 1987 Mar;384:603–618. doi: 10.1113/jphysiol.1987.sp016472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Kelly J. S., Leresche N., Pirchio M. The ventral and dorsal lateral geniculate nucleus of the rat: intracellular recordings in vitro. J Physiol. 1987 Mar;384:587–601. doi: 10.1113/jphysiol.1987.sp016471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Leresche N., Parnavelas J. G. Membrane properties of morphologically identified X and Y cells in the lateral geniculate nucleus of the cat in vitro. J Physiol. 1987 Sep;390:243–256. doi: 10.1113/jphysiol.1987.sp016697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysel U. T., Pape H. C., Van Schayck R. Contributions of inhibitory mechanisms to the shift responses of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1987 Jul;388:199–212. doi: 10.1113/jphysiol.1987.sp016610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourment A., Hirsch J. C., Marc M. E. Oscillations of the spontaneous slow-wave sleep rhythm in lateral geniculate nucleus relay neurons of behaving cats. Neuroscience. 1985 Apr;14(4):1061–1075. doi: 10.1016/0306-4522(85)90277-5. [DOI] [PubMed] [Google Scholar]
- Gabbott P. L., Somogyi J., Stewart M. G., Hámori J. GABA-immunoreactive neurons in the dorsal lateral geniculate nucleus of the rat: characterisation by combined Golgi-impregnation and immunocytochemistry. Exp Brain Res. 1986;61(2):311–322. doi: 10.1007/BF00239521. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale P. T., Sefton A. J., Baur L. A., Cottee L. J. Interrelations of the rat's thalamic reticular and dorsal lateral geniculate nuclei. Exp Brain Res. 1982;45(1-2):217–229. doi: 10.1007/BF00235781. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Ogren M. P., Vaughn J. E., Barber R. P., Wu J. Y. Light and electron microscopic immunocytochemical localization of glutamic acid decarboxylase in monkey geniculate complex: evidence for gabaergic neurons and synapses. J Neurosci. 1983 Jun;3(6):1245–1262. doi: 10.1523/JNEUROSCI.03-06-01245.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Godfraind J. M., Maruyama S. The presence and nature of inhibition in small slices of the dorsal lateral geniculate nucleus of rat and cat incubated in vitro. Brain Res. 1979 May 25;168(2):388–392. doi: 10.1016/0006-8993(79)90180-x. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Wheal H. V. The synaptically evoked late hyperpolarisation in hippocampal CA1 pyramidal cells is resistant to intracellular EGTA. Neuroscience. 1984 May;12(1):267–275. doi: 10.1016/0306-4522(84)90152-0. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. Neurons with presynaptic perikarya and presynaptic dendrites in the rat lateral geniculate nucleus. Brain Res. 1973 Sep 14;59:35–59. doi: 10.1016/0006-8993(73)90252-7. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987 Nov;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero V. M., Scott G. L. Synaptic terminals in the dorsal lateral geniculate nucleus from neurons of the thalamic reticular nucleus: a light and electron microscope autoradiographic study. Neuroscience. 1981;6(12):2561–2577. doi: 10.1016/0306-4522(81)90102-0. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Singer W. Ultrastructural identification of somata and neural processes immunoreactive to antibodies against glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the cat. Exp Brain Res. 1985;59(1):151–165. doi: 10.1007/BF00237675. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Singer W. Ultrastructure and synaptic relations of neural elements containing glutamic acid decarboxylase (GAD) in the perigeniculate nucleus of the cat. A light and electron microscopic immunocytochemical study. Exp Brain Res. 1984;56(1):115–125. doi: 10.1007/BF00237447. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara P. T., Lieberman A. R., Hunt S. P., Wu J. Y. Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; immunohistochemical studies by light and electron microscopy. Neuroscience. 1983;8(2):189–211. doi: 10.1016/0306-4522(83)90060-x. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984 Nov 1;229(3):374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- PURPURA D. P., SHOFER R. J. Intracellular recording from thalamic neurons during reticulocortical activation. J Neurophysiol. 1963 May;26:494–505. doi: 10.1152/jn.1963.26.3.494. [DOI] [PubMed] [Google Scholar]
- Roy J. P., Clercq M., Steriade M., Deschênes M. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol. 1984 Jun;51(6):1220–1235. doi: 10.1152/jn.1984.51.6.1220. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Lindström S. Amino acids in the dorsal lateral geniculate nucleus of the cat--collection in vivo. J Neurosci Methods. 1983 Sep;9(1):65–74. doi: 10.1016/0165-0270(83)90110-3. [DOI] [PubMed] [Google Scholar]
- Satou M., Mori K., Tazawa Y., Takagi S. F. Two types of postsynaptic inhibition in pyriform cortex of the rabbit: fast and slow inhibitory postsynaptic potentials. J Neurophysiol. 1982 Nov;48(5):1142–1156. doi: 10.1152/jn.1982.48.5.1142. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res. 1986;63(1):1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Sumitomo I., Nakamura M., Iwama K. Location and function of the so-called interneurons of rat lateral geniculate body. Exp Neurol. 1976 Apr;51(1):110–123. doi: 10.1016/0014-4886(76)90056-x. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H., Ayala G. F. A late increase in potassium conductance follows synaptic stimulation of granule neurons of the dentate gyrus. Neurosci Lett. 1982 Apr 26;29(3):243–248. doi: 10.1016/0304-3940(82)90324-x. [DOI] [PubMed] [Google Scholar]
- Wilson P. M. A photographic perspective on the origins, form, course and relations of the acetylcholinesterase-containing fibres of the dorsal tegmental pathway in the rat brain. Brain Res. 1985 Oct;357(2):85–118. doi: 10.1016/0165-0173(85)90001-3. [DOI] [PubMed] [Google Scholar]
- de Lima A. D., Montero V. M., Singer W. The cholinergic innervation of the visual thalamus: an EM immunocytochemical study. Exp Brain Res. 1985;59(1):206–212. doi: 10.1007/BF00237681. [DOI] [PubMed] [Google Scholar]


