Abstract
Hepatoid carcinoma is a rare malignancy defined as extrahepatic primary alpha‐fetoprotein‐producing carcinoma morphologically resembling hepatocellular carcinoma. It is extremely rare in the lungs, with ambiguous pathological descriptions and variable prognosis. Herein, we present the case of a 66‐year‐old man with a primary pulmonary hepatoid carcinoma in his right upper lung who received complete curative surgical resection and adjuvant chemotherapy. No signs of recurrence or distant metastasis have been observed for 57 months postoperation. In addition, the literature is reviewed and the pathological diagnostic pitfalls are discussed.
Keywords: Alpha‐fetoprotein, Hepatoid carcinoma, Lungs
Introduction
Hepatoid carcinoma is defined as a primary alpha‐fetoprotein (AFP)‐producing carcinoma composed of neoplastic cells morphologically resembling hepatocellular carcinoma [[1], [2], [3]]. Pulmonary hepatoid carcinoma is extremely rare, with less than 20 cases being reported. Herein, we present a case of primary pulmonary hepatoid carcinoma with description of complete clinical and pathological findings, outcome after surgical resection, and literature review.
Case report
A 66‐year‐old male presented with productive cough for 5 years. Recently the symptoms progressed with exertional dyspnea. There was a history of smoking one pack of cigarettes per day for 40 years. He had rheumatoid arthritis with regular medication and was a hepatitis B virus carrier for 30 years. He came to the hospital for health examination and a chest X‐ray showed a mass lesion at the right upper lung field with tracheal deviation to the left side (Fig. 1A). The AFP level was 8686 ng/mL, while the other tumor markers, such as squamous cell carcinoma antigen, carcinoembryonic antigen (CEA), and human chorionic gonadotropin were within normal ranges.
Figure 1.

(A) Chest radiograph demonstrated a right upper lobe mass (arrow). (B) Chest computed tomography demonstrated a soft tissue mass (arrow) measuring 7.3 cm in greatest dimension in the right upper lobe.
During admission no abnormality was found in the blood cell count and biochemistry measurements, such as levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and blood urea nitrogen‐to‐creatinine ratio. A chest computer tomography (CT) revealed a soft tissue mass measuring 7.3 × 5.6 × 3.3 cm over the right upper lobe of the lung with mediastinal lymphadenopathy (Fig. 1B). No definite focal lesion in the visible parts of liver parenchyma, spleen, pancreas, gallbladder, bilateral adrenal glands, and kidneys was observed. An abdominal sonography was performed, which showed few polyps in the gallbladder, but no abnormality was found either in the liver or stomach. Results of a brain CT and bone scan demonstrated no distant metastasis. The whole‐body positron emission tomography/CT showed a huge mass lesion with central photopenic uptake in the right upper lobe of the lung with suspicious mediastinal metastatic lymphadenopathy. The clinical staging was cT3N2M0. The patient received curative surgical resection on September 4, 2007.
During exploratory thoracotomy, a 12 × 6 × 6 cm mass over the right upper lobe with adhesion to the chest wall was found (Fig. 2A). Obstructive pneumonitis with abscess formation was noted in the region peripheral to the tumor. A right upper lobe lobectomy with the dissection of radical lymph nodes was performed.
Figure 2.
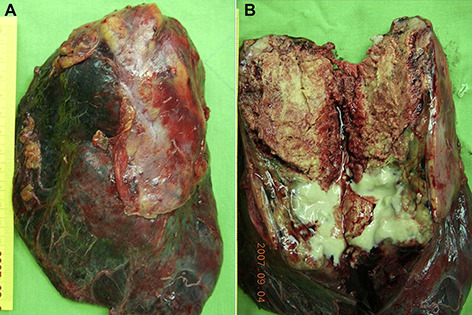
(A) Right upper lobe mass with adhesion to the chest wall. (B) The tumor measuring 7.4 × 6.0 × 4.8 cm showed central gray yellowish firm part with focal necrosis and consolidation in the periphery.
On gross examination, the tumor showed central yellowish gray firm part measuring 7.4 × 6.0 × 4.8 cm with focal necrosis and consolidation in the peripheral region (Fig. 2B). Visceral pleural invasion with chest wall soft tissue adhesion was found. A microscopic analysis showed that the tumor cells were arranged in predominantly trabecular and sheet‐like growth patterns (Fig. 3A) with rare pseudoglandular areas (Fig. 3B). No tubular or papillary adenocarcinoma was found in the extensively sampled specimen. The neoplastic cells were polygonal with large amount of eosinophilic cytoplasm and central hyperchromatic nuclei with prominent nucleoli, resembling hepatocellular carcinoma. There were frequent mitotic figures, up to 24 per 10 high power fields. Periodic acid–Schiff‐positive, diastase‐resistant hyaline globules were seen (Fig. 3C). Extensive necrosis is noted. The tumor invaded the chest wall, but all sampled lymph nodes were free of tumor involvement. The tumor cells were immunoreactive for cytokeratins (AE1/AE3), AFP (Fig. 3D), and glypican‐3 (Fig. 3E) diffusely, but negative for CK7, CK20, TTF‐1, hepatocyte paraffin 1 (Hep Par 1), and neuroendocrine markers, such as chromogranin A, synaptophysin, and CD56. Canalicular patterns were demonstrated by the immunohistochemical staining with polyclonal antibodies to CEA (pCEA) (Fig. 3F). No intracytoplasmic mucin vacuoles were demonstrated by the mucicarmine stain. The tumor was diagnosed as large cell carcinoma with prominent hepatoid differentiation, compatible with hepatoid carcinoma. The pathological staging was pT3N0M0, stage IIB.
Figure 3.

(A) Tumor cells arranged in predominantly trabecular and sheet‐like growth patterns, resembling hepatocellular carcinoma with focal necrosis. Hematoxylin and eosin stain (H&E), 400×. (B) Pseudoglandular pattern. H&E stain, 400×. (C) Periodic acid–Schiff (PAS)‐positive, diastase‐resistant hyaline globules. PAS stain, 400×. (D) Immunohistochemistry revealed diffusely positive alpha‐fetoprotein (AFP). AFP immunohistochemical stain, 400×. (E) The tumor cells are immunoreactive (positive) for glypican‐3. Glypican‐3 immunohistochemical stain, 400×. (F) Canalicular patterns demonstrated by the immunohistochemistry of polyclonal carcinoembryonic antigen (pCEA). pCEA immunohistochemical stain, 200×.
The patient received postoperative oral vinorelbine (Navelbine) and platin‐based chemotherapy for a period of 6 months. The AFP level dropped dramatically after the surgery and gradually decreased to normal level after the chemotherapy (Fig. 4). The patient received regular outpatient follow‐up and no local recurrence or distant metastasis was found after 4 years.
Figure 4.
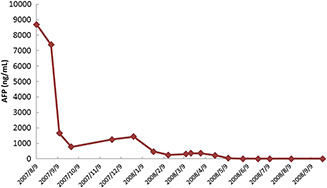
Level of serum alpha‐fetoprotein (AFP) level (surgery on September 4, 2007).
Discussion
Pulmonary hepatoid adenocarcinoma, first described by Ishikura et al., is defined as an AFP‐producing carcinoma containing a mixture of tubular or papillary adenocarcinoma with a sheet‐like or trabecular proliferation of neoplastic cells. The tumor cells contain abundant eosinophilic cytoplasm, centrally located nuclei, resembling those of hepatocellular carcinoma cells, as well as hyaline globules [4]. Histologically, the tubular or papillary component required for the diagnosis of adenocarcinoma was not present in our case. Instead, the hepatocellular‐like component including trabecular or sheet‐like growth patterns with evidence of AFP‐producing cells is essential for the histological diagnosis of hepatoid carcinoma. Although Ishikura et al. suggested to find the associated tubular/papillary adenocarcinomatous portion in a large cell carcinoma composed of large, eosinophilic cells with AFP production in order to fulfill the diagnostic criteria of hepatoid adenocarcinoma, we propose that the large cell carcinoma mainly composed of prominent AFP‐producing hepatoid component alone is qualified for the diagnosis of hepatoid carcinoma regardless of the adenocarcinomatous component, as described in several previous reports [[1], [2], [3]].
AFP is a fetal protein normally produced in liver, yolk sac, and gastrointestinal tract, but not in the lungs [5]. In adults, it is a tumor marker for liver cancer, yolk sac tumor, and certain gastrointestinal tumors. AFP production in the tumor cells is evident in all such reported cases by immunohistochemical analysis. In addition, expression of Hep Par 1, a monoclonal antibody considered almost specific for normal and neoplastic hepatocytes, had been demonstrated in hepatoid adenocarcinoma of the gastrointestinal tract [6], but only one previous study reported its expression in a pulmonary tumor [7]. Expression of glypican‐3, a placental and hepatic surface heparin sulfate proteoglycan expressed specifically in the fetal liver and malignant neoplasms of hepatocyte lineage, has not been studied in pulmonary hepatoid carcinoma previously. In AFP‐producing gastric carcinoma, glypican‐3 was suggested to be a sensitive marker for AFP‐producing gastric carcinomas and its hepatoid component [8]. Tumor cells of our case show diffuse glypican‐3 immunoreactivity, but its clinicopathological significance needs further study to clarify.
Clinically, exclusion of metastatic hepatocellular carcinoma or hepatoid carcinoma from extrahepatic sites is of crucial importance [4]. It is very difficult to differentiate when primary hepatoid carcinoma metastasizes to the liver, and therefore preoperative survey is important to obtain the diagnosis of primary pulmonary hepatoid carcinoma. Decreasing AFP level after the surgical resection of the primary tumor is significantly correlated with the subsequent management and follow‐up.
In the English literature, there are 14 cases of primary pulmonary hepatoid carcinoma and hepatoid adenocarcinoma without liver metastasis [[1], [2], [3], [4], [7], [9], [10], [11], [12]] (Table 1). All cases reported were male, with ages ranged from 35 to 82 years (mean age: 60 years). The tumor sizes range from 3.5 to 12 cm in the largest dimension with a mean size of 7.85 cm. Most cases are located at upper lung fields. In comprehensive review of the pathological descriptions of the reported cases, all cases contained hepatoid components with AFP‐producing cells, but seven cases including our case had no or scarce tubular/papillary adenocarcinomatous proportion. The AFP levels ranged from normal (two cases) to 160,000 ng/mL, but the AFP production was evident by immunohistochemical analysis in the cases with normal AFP level. Previously reported cases of hepatoid carcinoma showed poor prognosis [4]. However, recently reported cases [[1], [10], [11]] and our case that presented at an earlier stage when receiving surgery and/or chemotherapy had better prognosis. Pathological staging may be the most important prognostic factor in hepatoid carcinoma similar to the other nonsmall cell lung cancers.
Table 1.
Selected pulmonary hepatoid carcinomas in the literature.
| Case | Tumor site | Pathology review | Size (cm) | Serum AFP (ng/mL) | Stage | Prognosis | Reference (year) |
|---|---|---|---|---|---|---|---|
| 1 (67/M) | LUL | Ad | 8.0 | 160,000 | IV | 16 mo, D | Yasunami et al. [4] (1981) |
| 2 (66/M) | RLL | Large | 11.0 | 88,000 | IV | 6 mo, D | Yokoyama et al. [4] (1981) |
| 3 (40/M) | RUL | Ad | 9.0 | 3090 | IV | 14 mo, D | Miyake et al. [4] (1986) |
| 4 (55/M) | RUL | Ad | 5.0 | 2123 | IIIA | 4 d, D | Miyake et al. [4] (1986) |
| 5 (73/M) | LUL | Ad | 6.0 | 1039 | IV | 19 mo, D | Miyake et al. [4] (1987) |
| 6 (36/M) | LUL | Ad | 10.0 | 11,600 | IIIB | 7 mo, D | Arnould et al. [9] (1997) |
| 7 (63/M) | RUL | Large | 8.0 | 14,000 | IV | 11 mo, D | Nasu et al. [2] (1997) |
| 8 (82/M) | LLL | Large | 3.5 | Normal | IB | 7 y, A | Carlinfante et al. [1] (2000) |
| 9 (55/M) | RUL | Ad | 6.0 | 89 a | IIA | 30 mo, A | Hayashi et al. [10] (2002) |
| 10 (71/M) | RLL | Large | 10.5 | 9826 | IIIA | 12 mo, D | Hiroshima et al. [3] (2002) |
| 11 (50/M) | RUL | Large b | 6.0 | Normal | IIB | 45 mo, A | Wu et al. [11] (2007) |
| 12 (64/M) | RUL | Large | 7.5 | 673 | IIB | NA | Kishimoto et al. [7] (2008) |
| 13 (52/M) | LUL | Ad | 12 | 5000 | NA | 2 mo, A | Mokrim et al. [12] (2011) |
| 14 (66/M) | RUL | Large | 7.4 | 8868 | IIB | 4 y, A | Present case |
A = alive; ad = adenocarcinoma; D = died of disease; large = larger cell carcinoma; LLL = left lower lobe; LUL = left upper lobe; mo = months; NA = data not available; RLL = right lower lobe; RUL = right upper lobe; y = years.
Serum level after surgery.
No tubular or papillary adenocarcinomatous component described.
In conclusion, we have reported a case of primary pulmonary hepatoid carcinoma with typical clinicopathological features. The prognosis may be correlated with the pathological staging, but more data are needed to elucidate. We expect a fair survival in our present case after surgery and chemotherapy, while close follow‐up and monitor by AFP levels are recommended.
References
- [1]. Carlinfante G., Foschini M.P., Pasquinelli G., Scotti R., Cavazza A.. Hepatoid carcinoma of the lung: a case report with immunohistochemical, ultrastructural and in‐situ hybridization findings. Histopathology. 2000; 37: 88–89. [DOI] [PubMed] [Google Scholar]
- [2]. Nasu M., Soma T., Fukushima H., Kudo K., Matsubara O.. Hepatoid carcinoma of the lung with production of alpha‐fetoprotein and abnormal prothrombin: an autopsy case report. Mod Pathol. 1997; 10: 1054–1058. [PubMed] [Google Scholar]
- [3]. Hiroshima K., Iyoda A., Toyozaki T., Haga Y., Baba M., Fujisawa T., et al. Alpha‐fetoprotein‐producing lung carcinoma: report of three cases. Pathol Int. 2002; 52: 46–53. [DOI] [PubMed] [Google Scholar]
- [4]. Ishikura H., Kanda M., Ito M., Nosaka K., Mizuno K.. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha‐fetoprotein‐producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990; 417: 73–80. [DOI] [PubMed] [Google Scholar]
- [5]. Gitlin D., Perricelli A., Gitlin G.M.. Synthesis of alpha‐fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972; 32: 979–982. [PubMed] [Google Scholar]
- [6]. Maitra A., Murakata L.A., Albores‐Saavedra J.. Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol. 2001; 115: 689–694. [DOI] [PubMed] [Google Scholar]
- [7]. Kishimoto T., Yano T., Hiroshima K., Inayama Y., Kawachi K., Nakatani Y.. A case of alpha‐fetoprotein‐producing pulmonary carcinoma with restricted expression of hepatocyte nuclear factor‐4alpha in hepatoid foci: a case report with studies of previous cases. Hum Pathol. 2008; 39: 1115–1120. [DOI] [PubMed] [Google Scholar]
- [8]. Hishinuma M., Ohashi K.I., Yamauchi N., Kashima T., Uozaki H., Ota S., et al. Hepatocellular oncofetal protein, glypican 3 is a sensitive marker for alpha‐fetoprotein‐producing gastric carcinoma. Histopathology. 2006; 49: 479–486. [DOI] [PubMed] [Google Scholar]
- [9]. Arnould L., Drouot F., Fargeot P., Bernard A., Foucher P., Collin F., et al. Hepatoid adenocarcinoma of the lung: report of a case of an unusual alpha‐fetoprotein‐producing lung tumor. Am J Surg Pathol. 1997; 21: 1113–1118. [DOI] [PubMed] [Google Scholar]
- [10]. Hayashi Y., Takanashi Y., Ohsawa H., Ishii H., Nakatani Y.. Hepatoid adenocarcinoma in the lung. Lung Cancer. 2002; 38: 211–214. [DOI] [PubMed] [Google Scholar]
- [11]. Wu Z., Upadhyaya M., Zhu H., Qiao Z., Chen K., Miao F.. Hepatoid adenocarcinoma: computed tomographic imaging findings with histopathologic correlation in 6 cases. J Comput Assist Tomogr. 2007; 31: 846–852. [DOI] [PubMed] [Google Scholar]
- [12]. Mokrim M., Belbaraka R., Allaoui M., Kairaouani M., Mahassini N., Tahri A., et al. Hepatoid adenocarcinoma of the lung: a case report and literature review. J Gastrointest Cancer. 2012; 43 (Suppl 1): 125–127. [DOI] [PubMed] [Google Scholar]


