Abstract
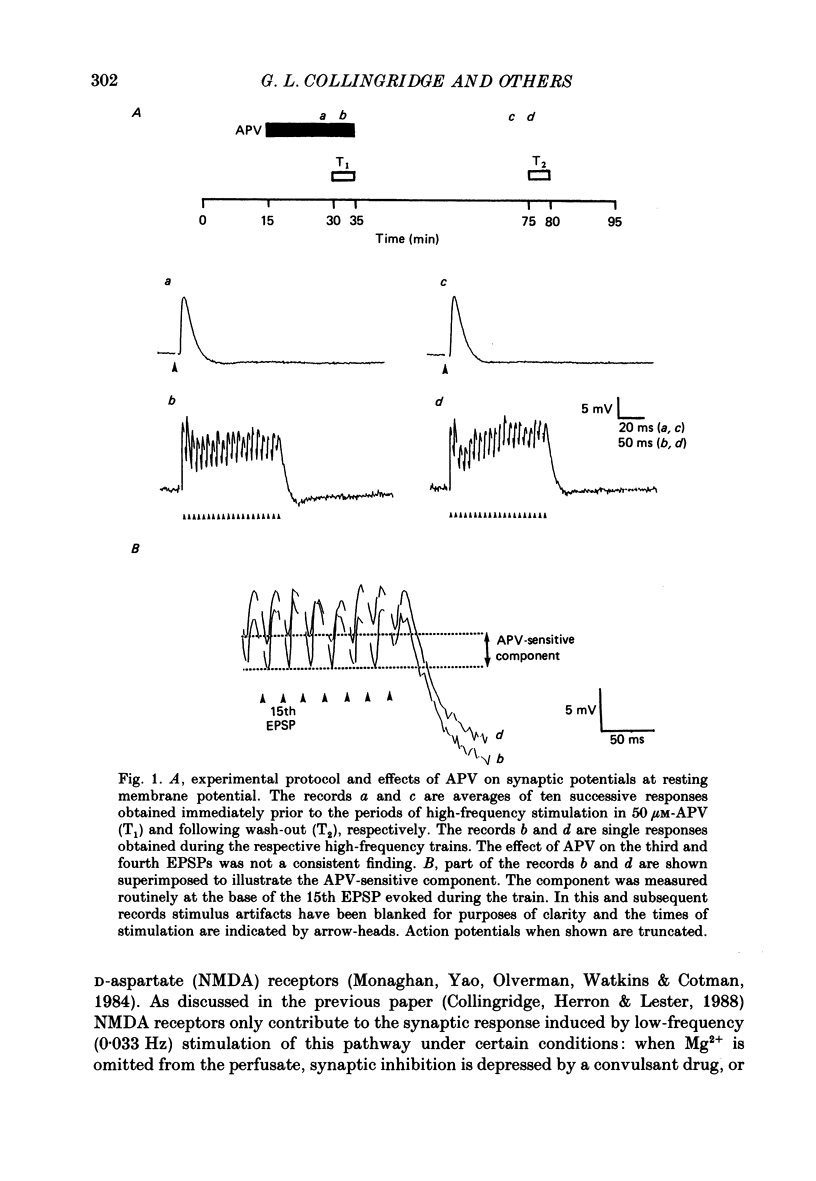
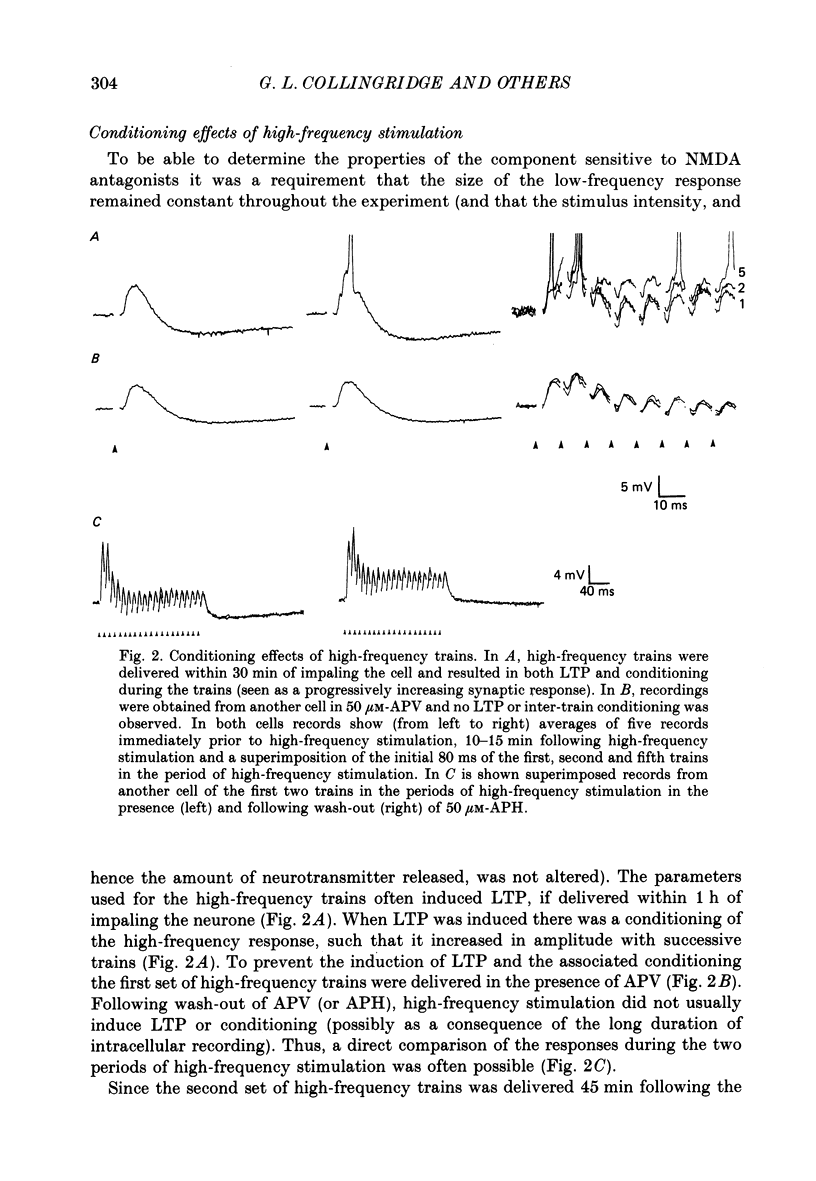
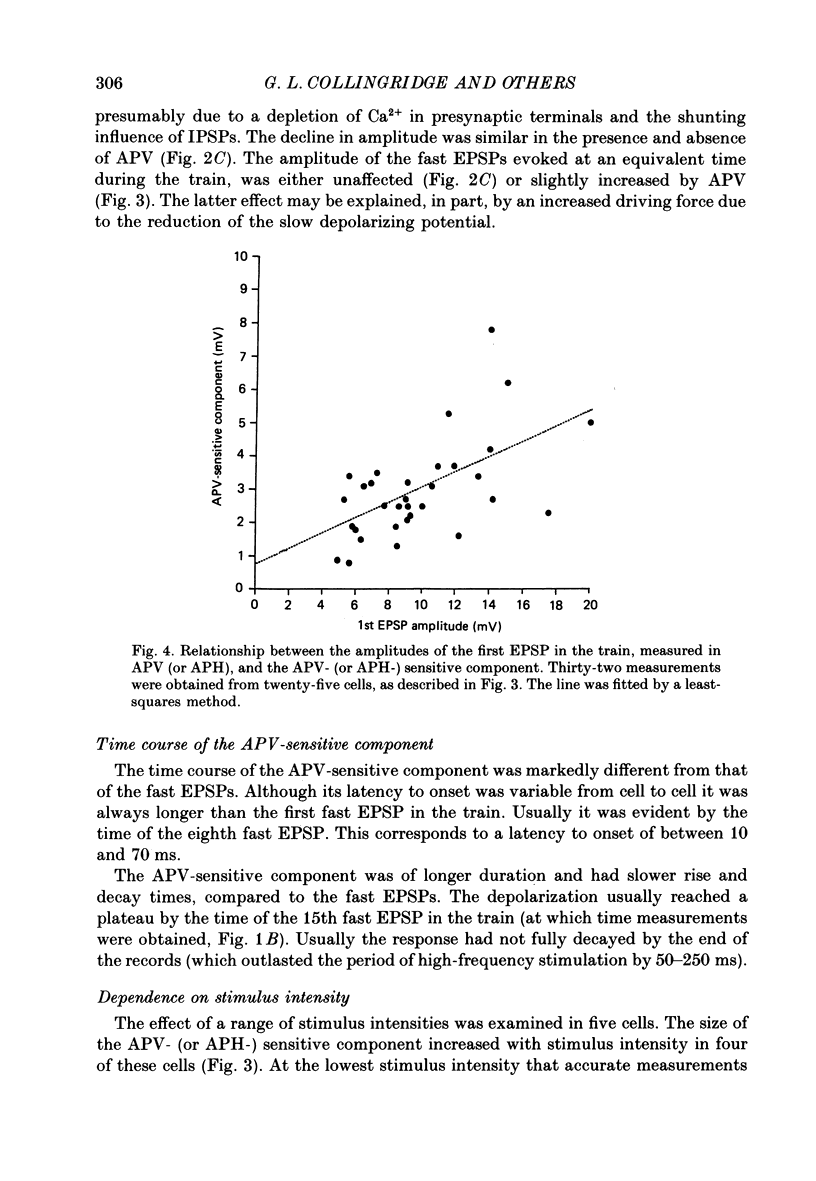
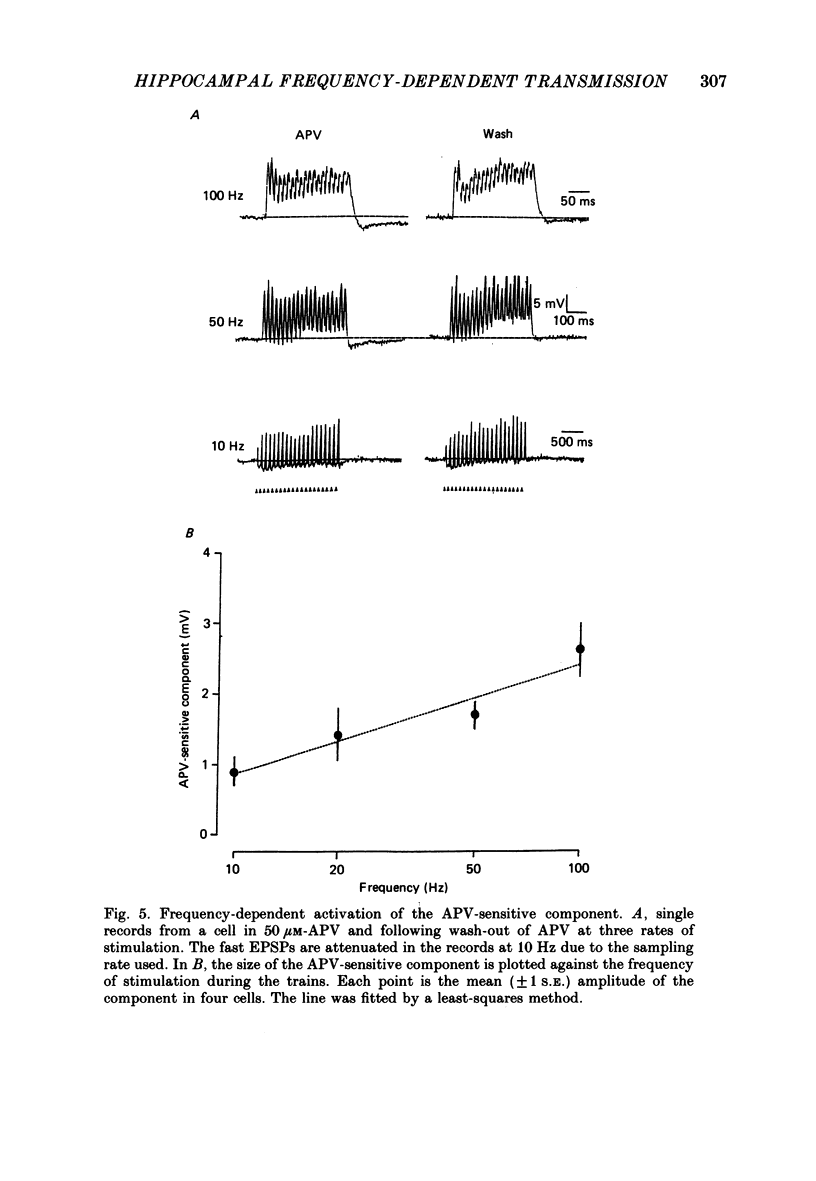
1. The effects of the N-methyl-D-aspartate (NMDA) antagonist, D-2-amino-5-phosphonovalerate (APV) were examined on synaptic responses evoked by high-frequency stimulation of the Schaffer collateral-commissural pathway, in the presence of Mg2+ (1 or 2 mM) and functional synaptic inhibition. 2. The synaptic response evoked by 100 Hz stimulation comprised fast excitatory postsynaptic potentials (EPSPs) evoked by each shock and a slow depolarization. APV reduced the size of the depolarization without depressing the fast EPSPs. 3. The mean (+/- 1 S.E.) amplitude of the APV-sensitive component (3.0 +/- 0.3 mV), evoked by 100 Hz stimulation at membrane potentials near rest, was invariably smaller than the first fast EPSP (9.8 +/- 0.7 mV). Both of these synaptic components had similar thresholds and increased in amplitude as the stimulus intensity was raised. There was a positive correlation between the amplitude of the two components (r = 0.57, P less than 0.01). 4. The amplitude of the APV-sensitive component was positively correlated (r = 0.97, P less than 0.05) with the frequency of stimulation during the trains (between 10 and 100 Hz). The threshold frequency for evoking an APV-sensitive component was approximately 10 Hz. 5. In contrast to the fast EPSPs the amplitude of the APV-sensitive component increased with depolarization, and decreased with hyperpolarization, of a neurone from its resting membrane potential. The component was no longer present in some cells which had been hyperpolarized sufficiently. 6. It is suggested that during high-frequency stimulation a neurone may become depolarized for a sufficient time to reduce the Mg2+ block of NMDA channels. This enables the NMDA receptor system to contribute transiently to the synaptic response, despite the inhibitory synaptic mechanisms which prevent its activation during single-shock stimulation. The characteristics of the NMDA receptor-mediated synaptic response may explain properties relating to the induction of long-term potentiation (LTP).
Full text
PDF


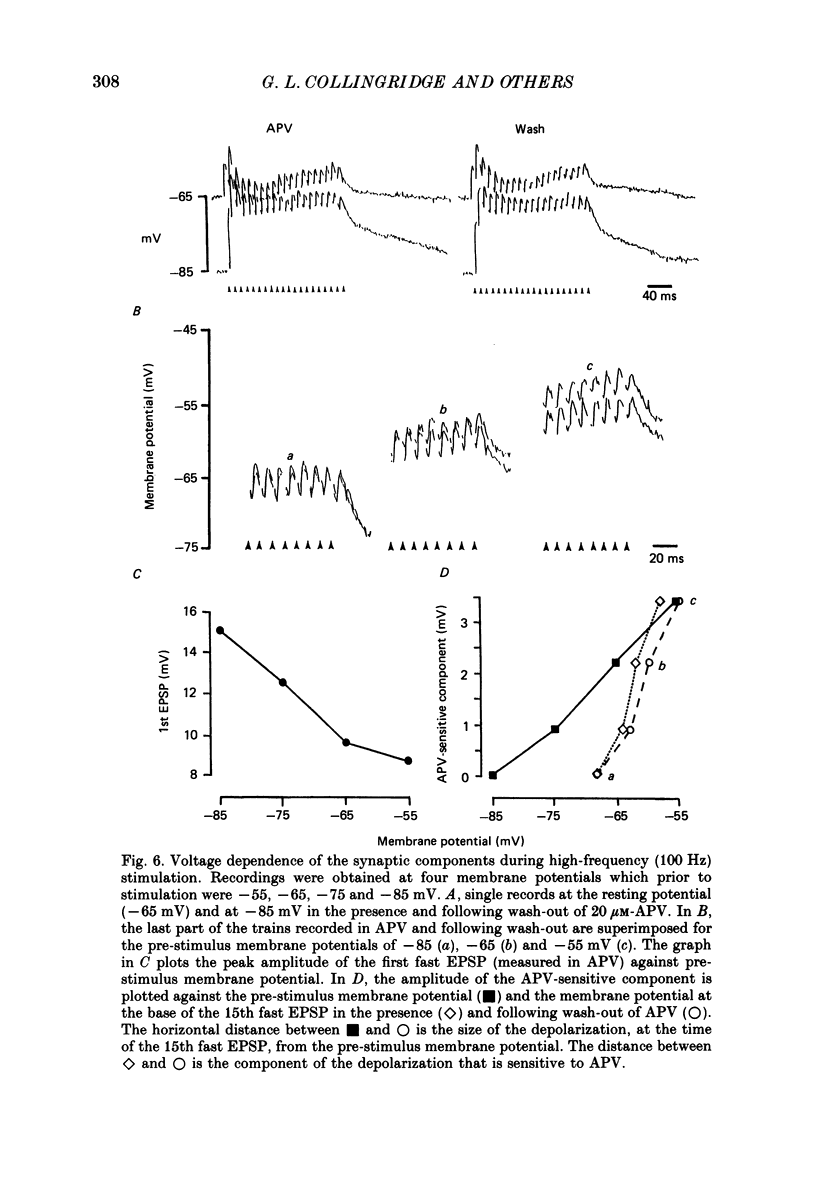




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benninger C., Kadis J., Prince D. A. Extracellular calcium and potassium changes in hippocampal slices. Brain Res. 1980 Apr 7;187(1):165–182. doi: 10.1016/0006-8993(80)90502-8. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. J Physiol. 1983 Oct;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V., Riives M. Inhibitory modulation of long-term potentiation: evidence for a postsynaptic locus of control. Brain Res. 1982 May 27;240(2):259–272. doi: 10.1016/0006-8993(82)90221-9. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T., Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978 Mar;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Williamson R., Collingridge G. L. A selective N-methyl-D-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neurosci Lett. 1985 Nov 11;61(3):255–260. doi: 10.1016/0304-3940(85)90473-2. [DOI] [PubMed] [Google Scholar]
- Kelso S. R., Ganong A. H., Brown T. H. Hebbian synapses in hippocampus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Malinow R., Miller J. P. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986 Apr 10;320(6062):529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarren M., Alger B. E. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985 Feb;53(2):557–571. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Olverman H. J., Watkins J. C., Cotman C. W. Autoradiography of D-2-[3H]amino-5-phosphonopentanoate binding sites in rat brain. Neurosci Lett. 1984 Dec 21;52(3):253–258. doi: 10.1016/0304-3940(84)90170-8. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sastry B. R., Goh J. W., Auyeung A. Associative induction of posttetanic and long-term potentiation in CA1 neurons of rat hippocampus. Science. 1986 May 23;232(4753):988–990. doi: 10.1126/science.3010459. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Stelzer A., Galvan M. Kindling-like stimulus patterns induce epileptiform discharges in the guinea pig in vitro hippocampus. Neurosci Lett. 1985 Sep 16;60(1):25–31. doi: 10.1016/0304-3940(85)90376-3. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. A possible correlate of the postsynaptic condition for long-lasting potentiation in the guinea pig hippocampus in vitro. Neurosci Lett. 1984 Feb 24;44(3):327–332. doi: 10.1016/0304-3940(84)90044-2. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985 Sep;125(1):159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]


