Abstract
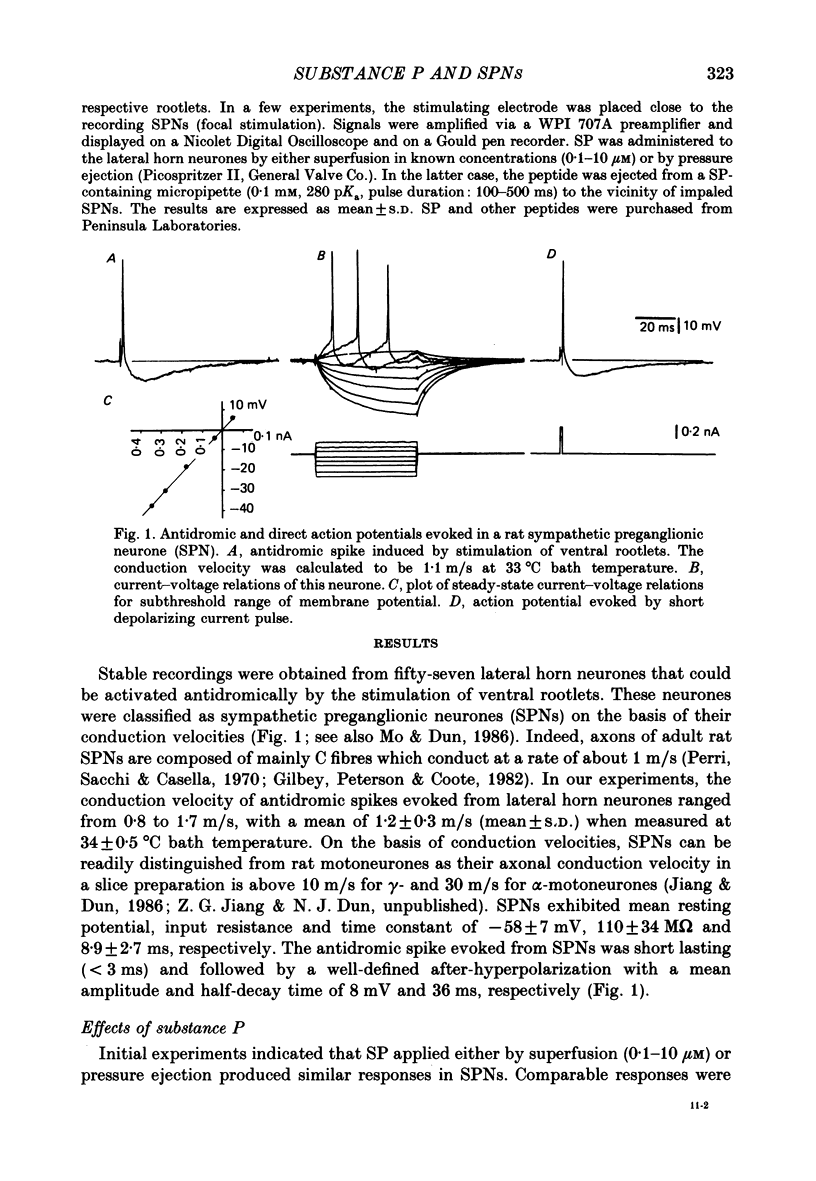
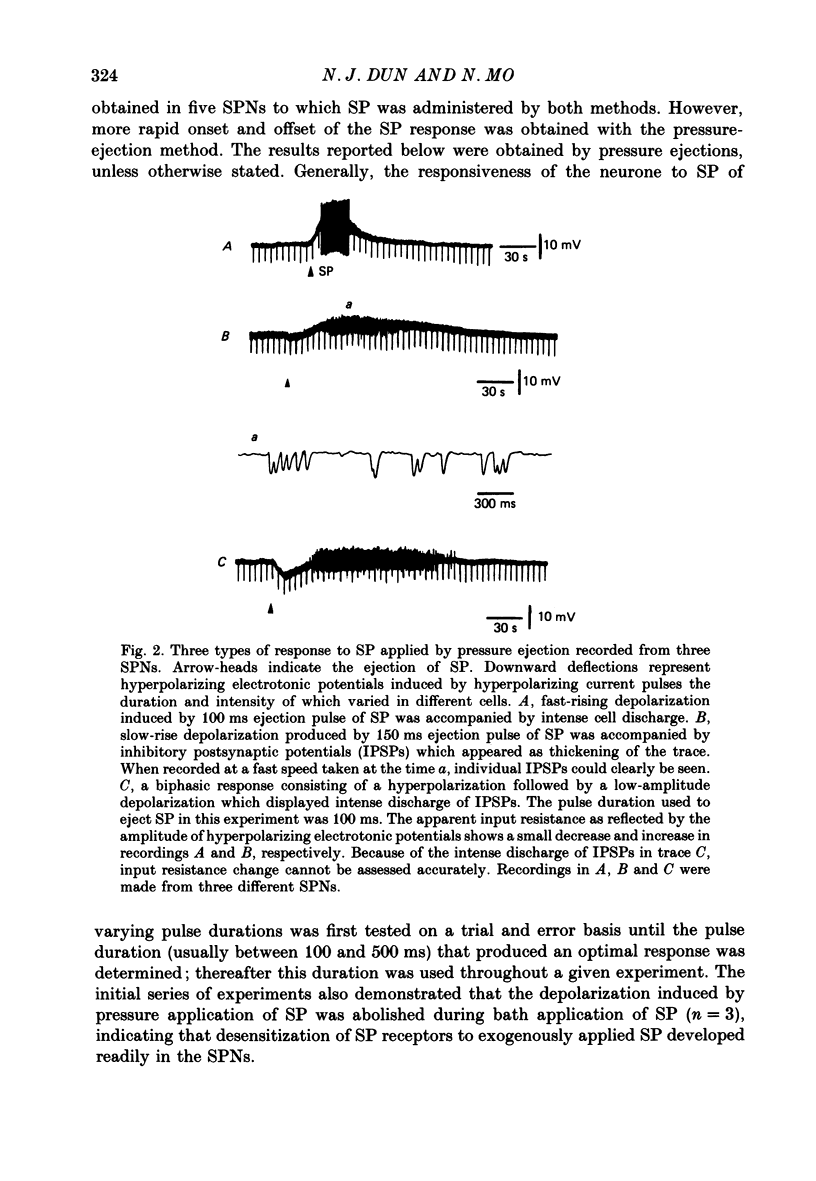
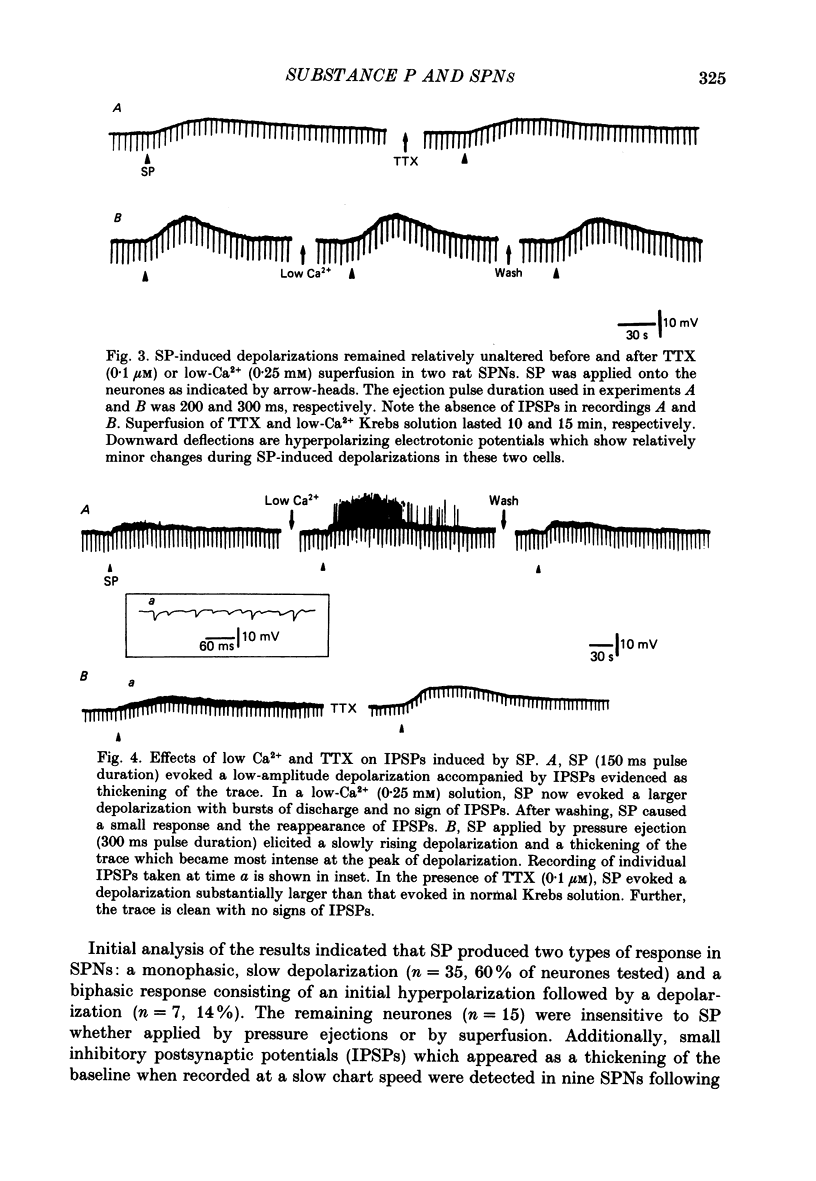
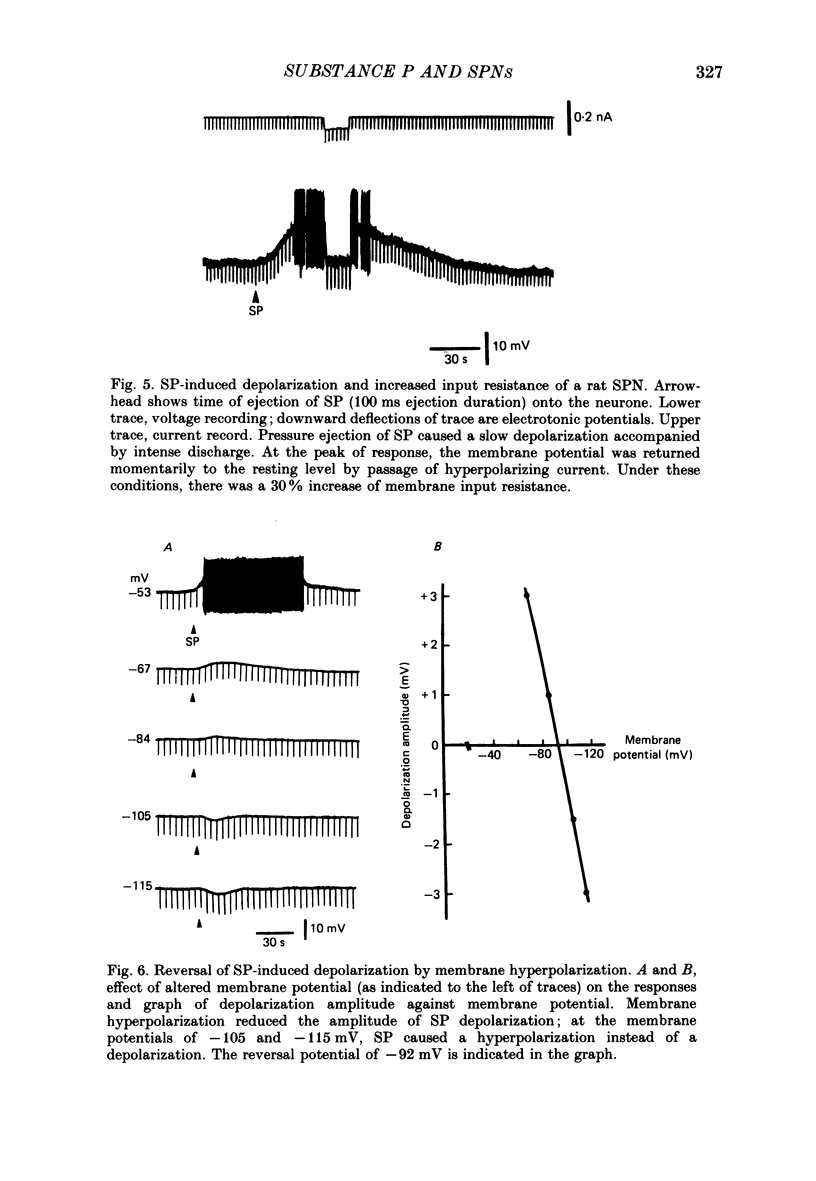
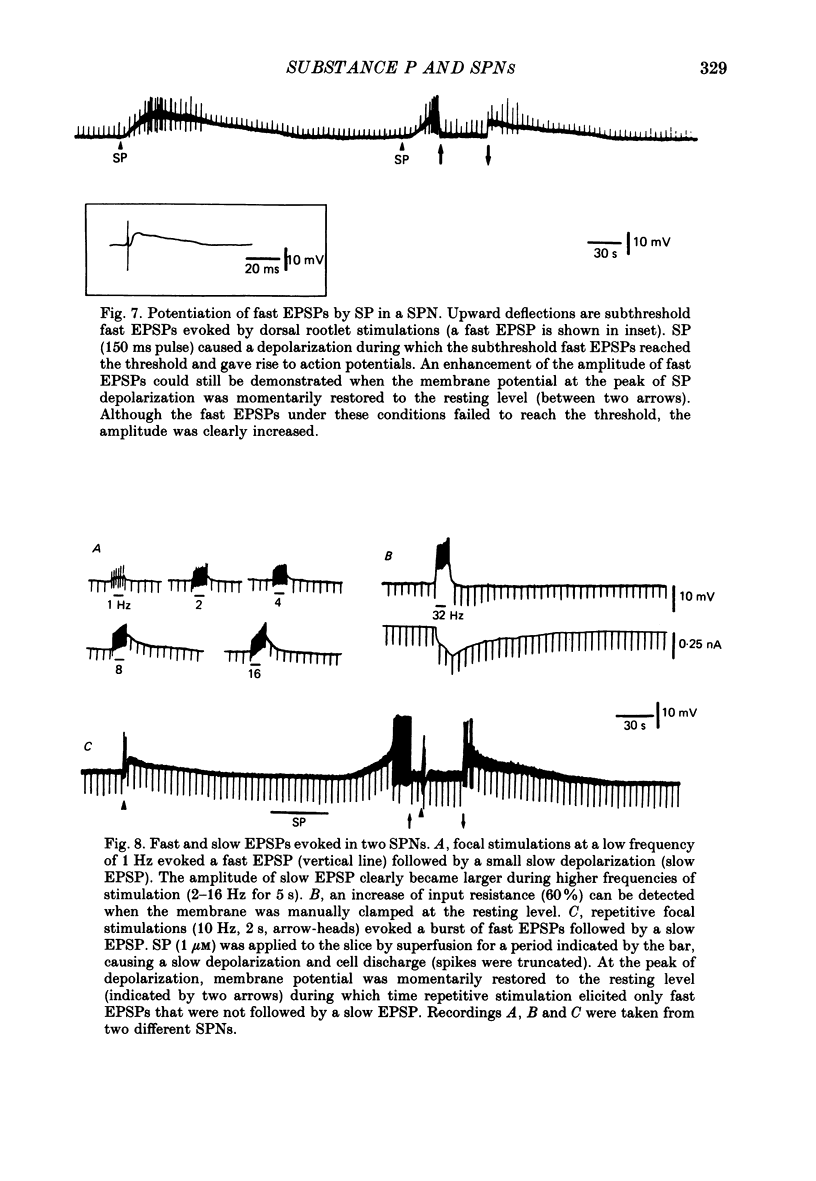
1. Intracellular recordings were made from antidromically identified sympathetic preganglionic neurones (SPNs) in thin transverse neonatal rat thoracolumbar spinal cord slices. 2. Applied either by pressure ejection or superfusion, substance P (SP) caused a slow, monophasic depolarization in 60% of sympathetic preganglionic neurones; a biphasic response consisting of an initial hyperpolarization followed by a depolarization was observed in a few neurones. In addition, SP induced the occurrence of repetitive inhibitory postsynaptic potentials (IPSPs) in about 20% SPNs. 3. Low-Ca2+ or tetrodotoxin (TTX)-containing Krebs solution abolished the hyperpolarizing phase of the biphasic response and the small IPSPs, thereby augmenting the depolarizing response of SP. 4. SP-induced depolarizations were often associated with a moderate increase in membrane resistance. Generally, the response was made smaller on hyperpolarization and reversed at the membrane potential between -90 and -100 mV. These findings suggest that a reduction of membrane K+ conductance may underlie the depolarizing action of SP. 5. Subthreshold fast, excitatory postsynaptic potentials (EPSPs) evoked by stimulation of dorsal rootlets were consistently augmented during SP-induced depolarization, leading to cell discharge. 6. Focal stimulations elicited, in addition to a fast EPSP, a slow EPSP in about 40% of SPNs. The slow EPSP was often associated with an increased membrane resistance and became smaller on hyperpolarization. 7. In 15% of SPNs that generated a slow EPSP, the latter was reversibly abolished during SP-induced depolarization; the blockade persisted when the membrane potential was restored to the resting level by hyperpolarizing current. 8. It is concluded that SP is excitatory to SPNs and that its synaptic release may initiate a slow EPSP which serves to augment impulse transmission through SPNs. Further, it appears that inhibitory interneurones may also be sensitive to SP and their activation may provide a negative feed-back mechanism which can limit excessive excitation of SPNs by the peptide.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Jones S. W. Substance P inhibits the M-current in bullfrog sympathetic neurones. Br J Pharmacol. 1983 Jun;79(2):330–333. doi: 10.1111/j.1476-5381.1983.tb11004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi H., Konishi S., Otsuka M., Yanagisawa M. The role of substance P as a neurotransmitter in the reflexes of slow time courses in the neonatal rat spinal cord. Br J Pharmacol. 1985 Mar;84(3):663–673. doi: 10.1111/j.1476-5381.1985.tb16148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman S. B., Henry J. L. Effect of substance P and thyrotropin-releasing hormone on sympathetic preganglionic neurones in the upper thoracic intermediolateral nucleus of the cat. Can J Physiol Pharmacol. 1984 Feb;62(2):248–251. doi: 10.1139/y84-038. [DOI] [PubMed] [Google Scholar]
- Charlton C. G., Helke C. J. Autoradiographic localization and characterization of spinal cord substance P binding sites: high densities in sensory, autonomic, phrenic, and Onuf's motor nuclei. J Neurosci. 1985 Jun;5(6):1653–1661. doi: 10.1523/JNEUROSCI.05-06-01653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello A. C., Kanazawa I. The distribution of substance P immunoreactive fibers in the rat central nervous system. J Comp Neurol. 1978 Mar 1;178(1):129–156. doi: 10.1002/cne.901780108. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res. 1971 Jun 29;12(5):547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G. Non-cholinergic excitatory transmission in inferior mesenteric ganglia of the guinea-pig: possible mediation by substance P. J Physiol. 1982 Apr;325:145–159. doi: 10.1113/jphysiol.1982.sp014141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey M. P., McKenna K. E., Schramm L. P. Effects of substance P on sympathetic preganglionic neurones. Neurosci Lett. 1983 Oct 31;41(1-2):157–159. doi: 10.1016/0304-3940(83)90239-2. [DOI] [PubMed] [Google Scholar]
- Gilbey M. P., Peterson D. F., Coote J. H. Some characteristics of sympathetic preganglionic neurones in the rat. Brain Res. 1982 Jun 3;241(1):43–48. doi: 10.1016/0006-8993(82)91226-4. [DOI] [PubMed] [Google Scholar]
- Helke C. J., Neil J. J., Massari V. J., Loewy A. D. Substance P neurons project from the ventral medulla to the intermediolateral cell column and ventral horn in the rat. Brain Res. 1982 Jul 8;243(1):147–152. doi: 10.1016/0006-8993(82)91128-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Jiang Z. G., Dun N. J. Presynaptic suppression of excitatory postsynaptic potentials in rat ventral horn neurons by muscarinic agonists. Brain Res. 1986 Aug 27;381(1):182–186. doi: 10.1016/0006-8993(86)90710-9. [DOI] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Keeler J. R., Charlton C. G., Helke C. J. Cardiovascular effects of spinal cord substance P: studies with a stable receptor agonist. J Pharmacol Exp Ther. 1985 Jun;233(3):755–760. [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl A., Hökfelt T., Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3(10):861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Sawyer W. B. Substance P antagonist inhibits vasomotor responses elicited from ventral medulla in rat. Brain Res. 1982 Aug 12;245(2):379–383. doi: 10.1016/0006-8993(82)90822-8. [DOI] [PubMed] [Google Scholar]
- Ma R. C., Dun N. J. Excitation of lateral horn neurons of the neonatal rat spinal cord by 5-hydroxytryptamine. Brain Res. 1986 Jan;389(1-2):89–98. doi: 10.1016/0165-3806(86)90176-8. [DOI] [PubMed] [Google Scholar]
- Ma R. C., Dun N. J. Vasopressin depolarizes lateral horn cells of the neonatal rat spinal cord in vitro. Brain Res. 1985 Nov 25;348(1):36–43. doi: 10.1016/0006-8993(85)90356-7. [DOI] [PubMed] [Google Scholar]
- Maurin Y., Buck S. H., Wamsley J. K., Burks T. F., Yamamura H. I. Light microscopic autoradiographic localization of [3H] substance P binding sites in rat thoracic spinal cord. Life Sci. 1984 Apr 30;34(18):1713–1716. doi: 10.1016/0024-3205(84)90569-1. [DOI] [PubMed] [Google Scholar]
- McLennan H. Receptors for the excitatory amino acids in the mammalian central nervous system. Prog Neurobiol. 1983;20(3-4):251–271. doi: 10.1016/0301-0082(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Mo N., Dun N. J. Excitatory postsynaptic potentials in neonatal rat sympathetic preganglionic neurons: possible mediation by NMDA receptors. Neurosci Lett. 1987 Jun 26;77(3):327–332. doi: 10.1016/0304-3940(87)90522-2. [DOI] [PubMed] [Google Scholar]
- Mo N., Dun N. J. Is glycine an inhibitory transmitter in rat lateral horn cells? Brain Res. 1987 Jan 1;400(1):139–144. doi: 10.1016/0006-8993(87)90661-5. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Actions of substance P on rat spinal dorsal horn neurones. J Physiol. 1984 Jan;346:203–217. doi: 10.1113/jphysiol.1984.sp015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randić M. Substance P augments a persistent slow inward calcium-sensitive current in voltage-clamped spinal dorsal horn neurons of the rat. Brain Res. 1986 Feb 19;365(2):369–376. doi: 10.1016/0006-8993(86)91652-5. [DOI] [PubMed] [Google Scholar]
- Nowak L. M., Macdonald R. L. Substance P: ionic basis for depolarizing responses of mouse spinal cord neurons in cell culture. J Neurosci. 1982 Aug;2(8):1119–1128. doi: 10.1523/JNEUROSCI.02-08-01119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Konishi S., Takahashi T. Hypothalamic substance P as a candidate for transmitter of primary afferent neurons. Fed Proc. 1975 Sep;34(10):1922–1928. [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Takano Y., Sawyer W. B., Sanders N. L., Loewy A. D. LH-RH analogue acts as substance P antagonist by inhibiting spinal cord vasomotor responses. Brain Res. 1985 Jul 1;337(2):357–361. doi: 10.1016/0006-8993(85)90075-7. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]


