Abstract
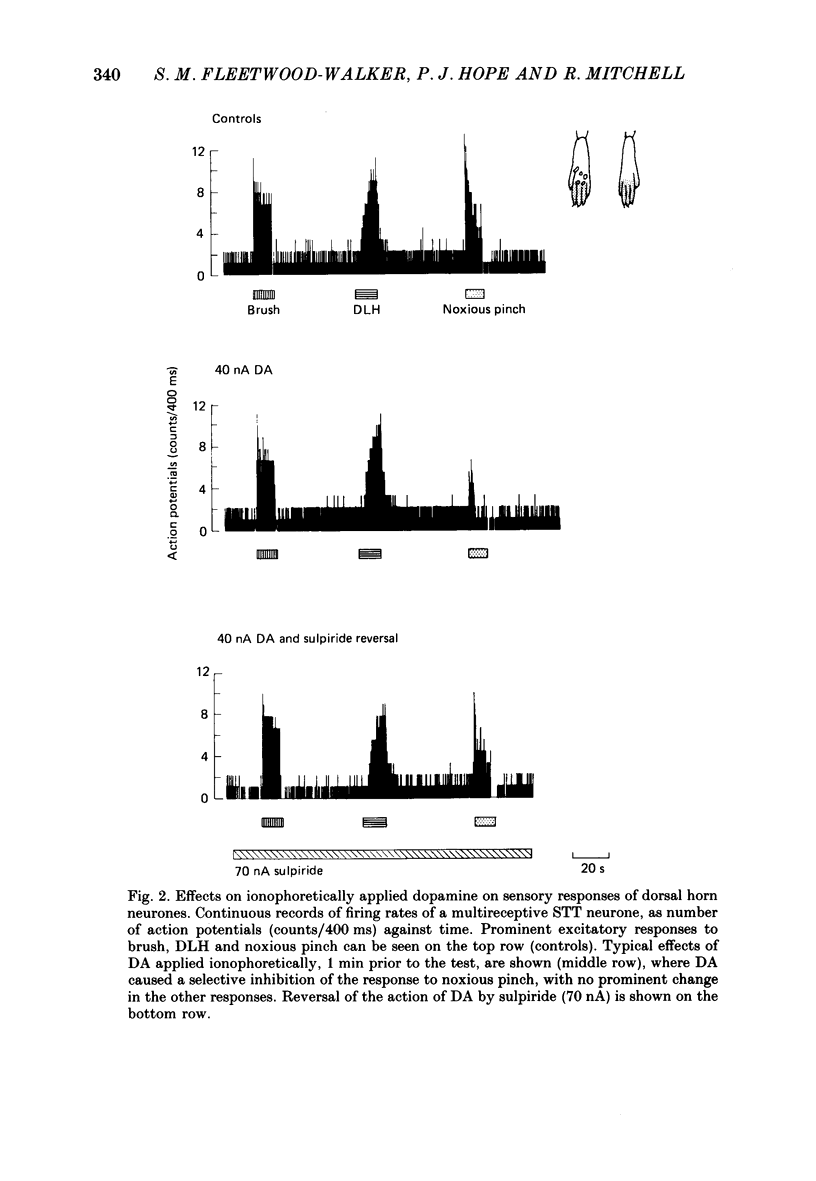
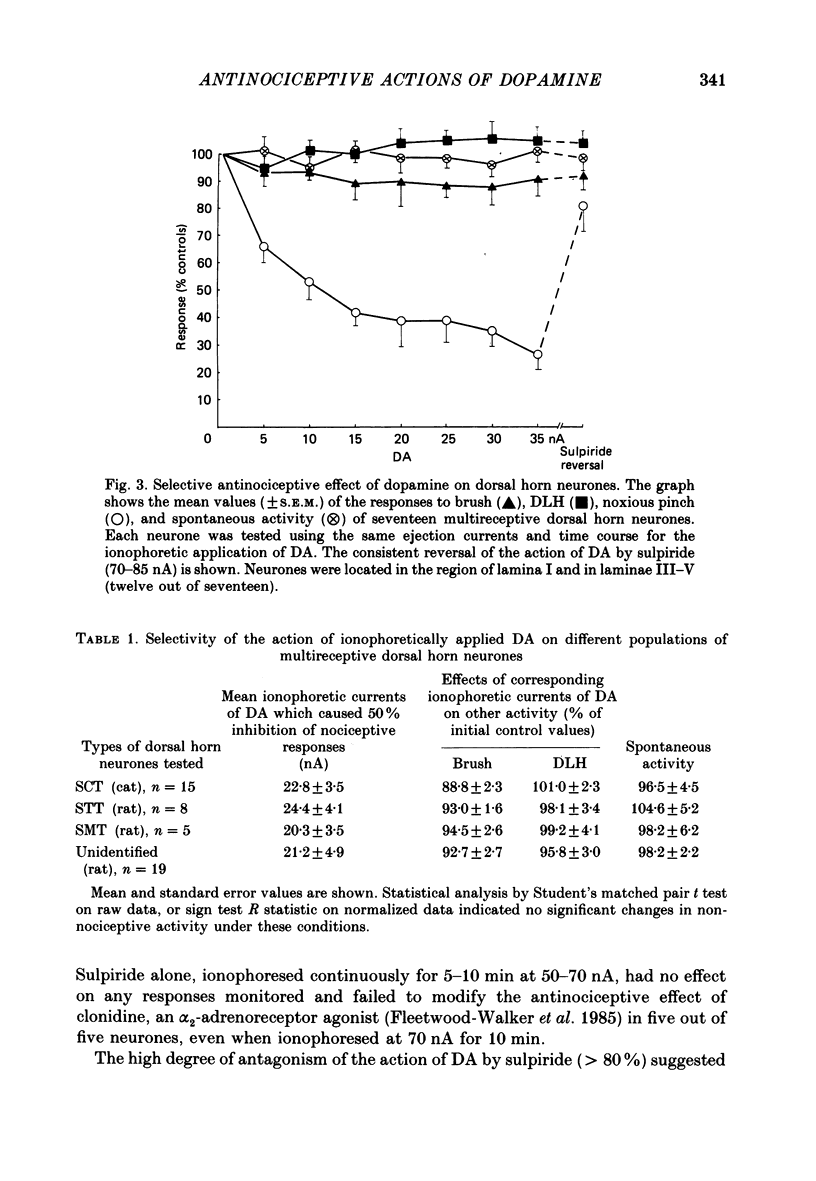
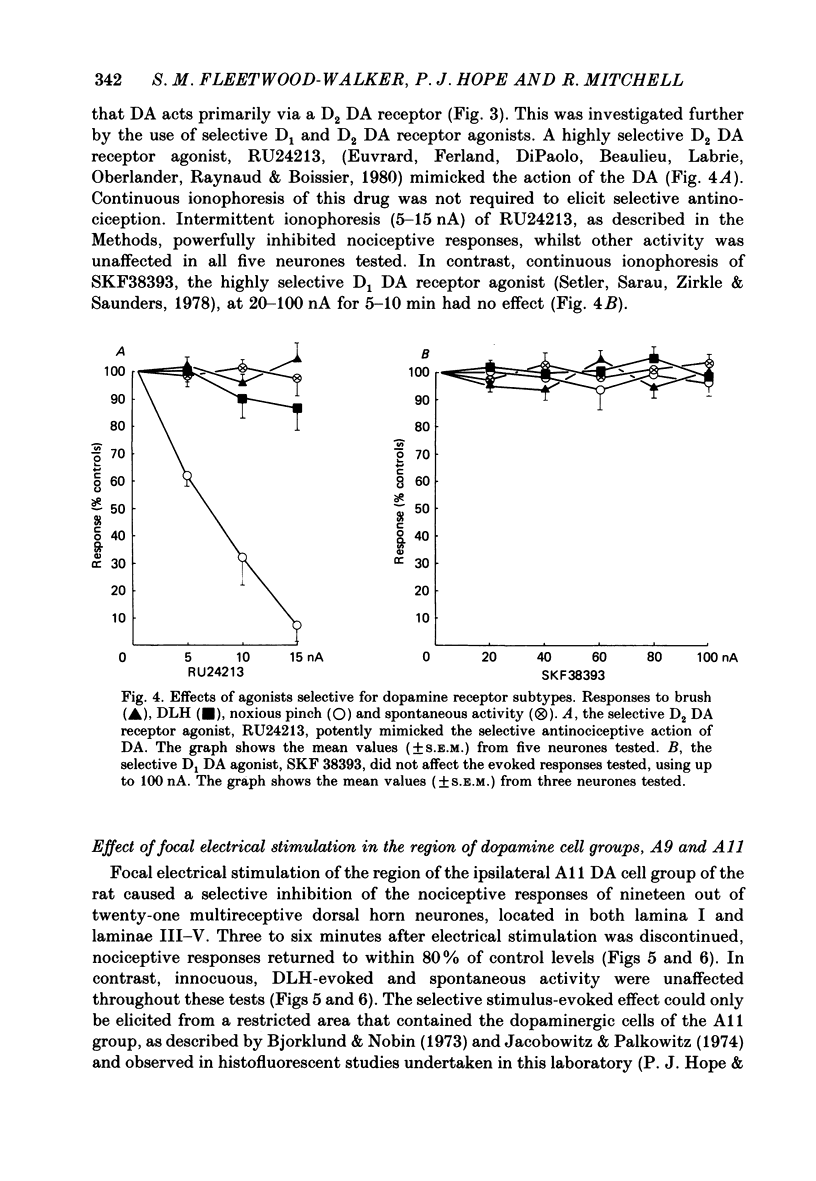
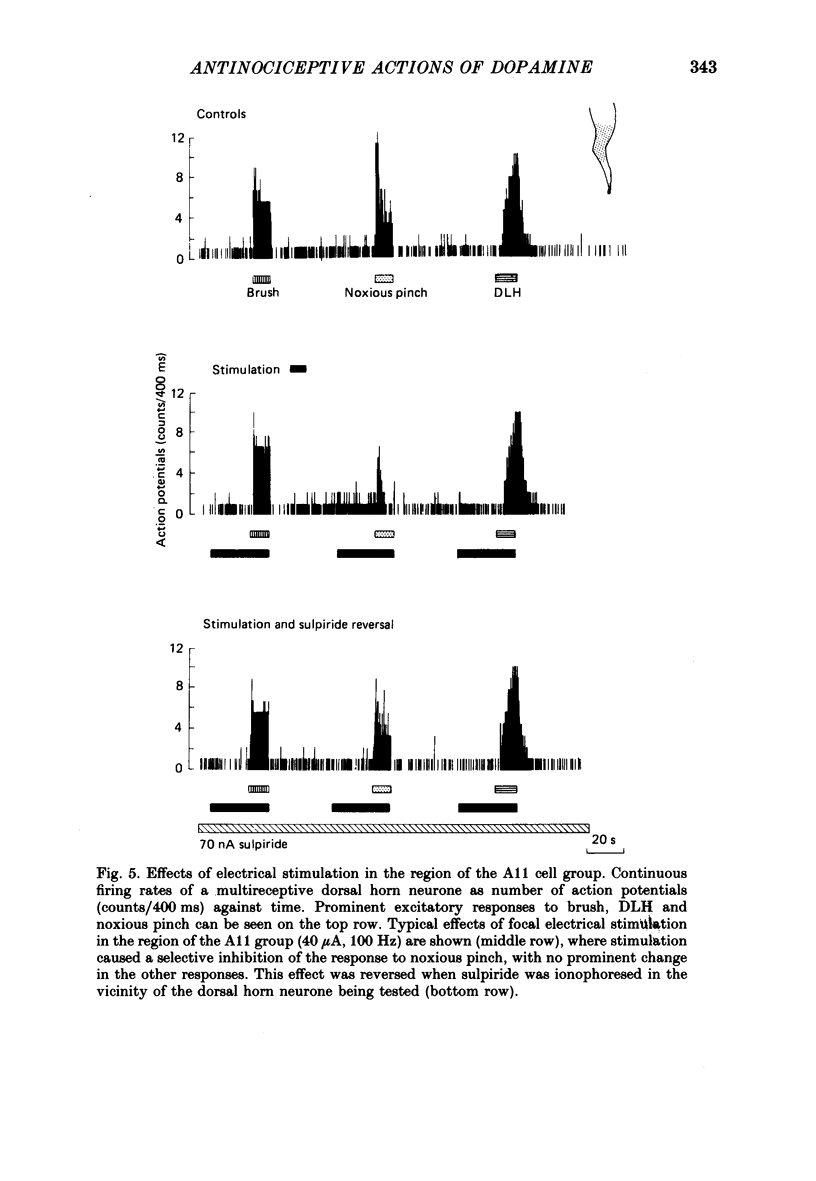
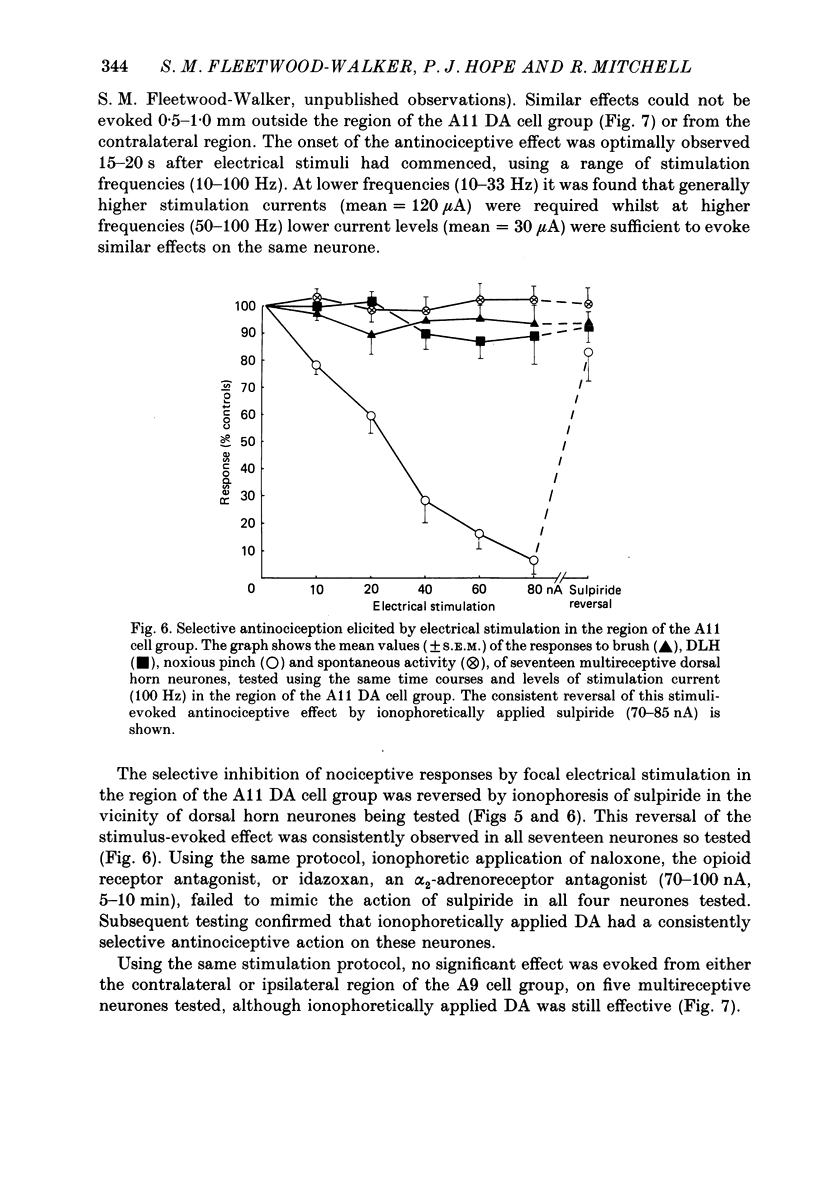
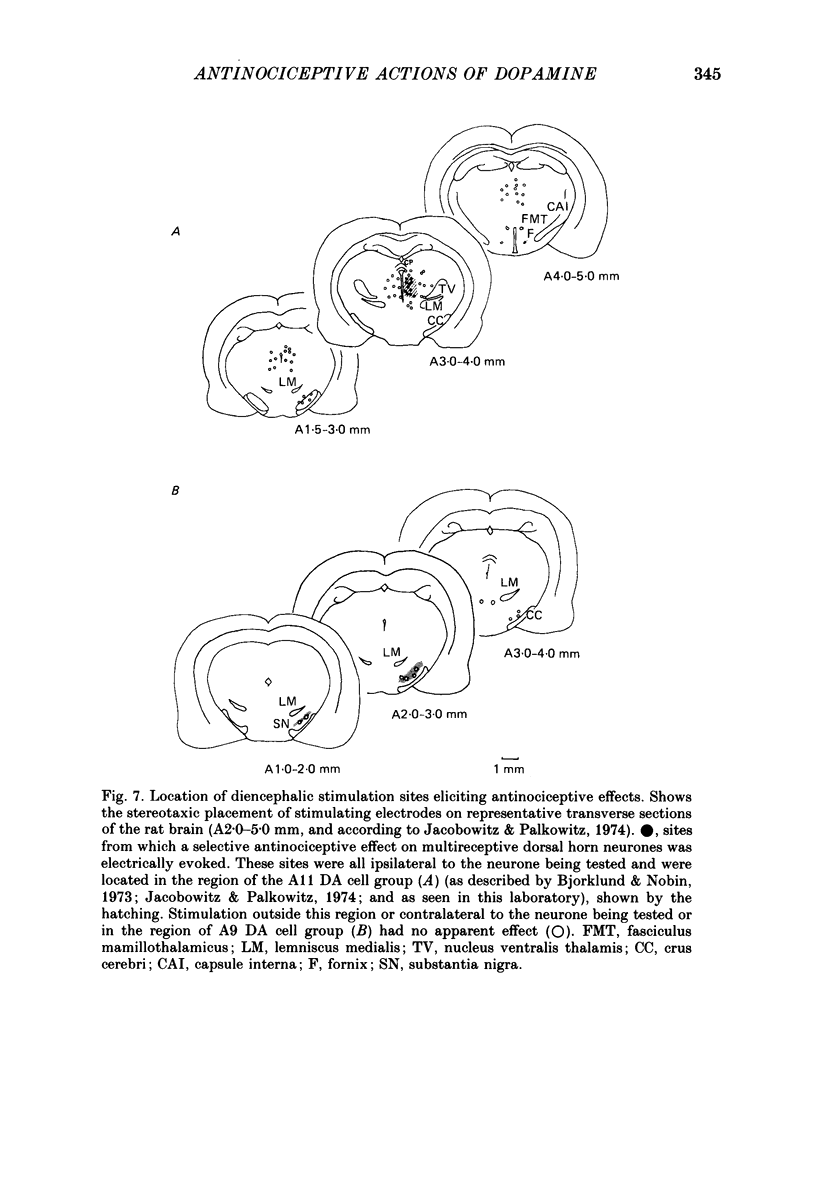
1. The actions of dopamine (DA) and DA receptor specific agonists and antagonist ionophoretically applied in the spinal dorsal horn, and of focal electrical stimulation in the region of the supraspinal DA cell groups (A9 and A11) were assessed on the somatosensory responses of dorsal horn neurones, in both the rat and cat. The neurones tested were multireceptive, giving reproducible responses to both noxious (using a mechanical pinch or radiant heat) and innocuous (using a motorized brush) cutaneous stimuli, as well as to ionophoretically applied DL-homocysteic acid (DLH, a direct excitant). In the cat, all neurones tested were identified as belonging to the spinocervical tract (SCT) and were located in the dorsal horn laminae III-V, whilst in the rat, spinothalamic tract (STT) and spinomesencephalic (SMT) neurones located in the region of lamina I and laminae III-V were tested. 2. Ionophoretically applied DA and RU24213, a D2 DA receptor agonist, caused a selective inhibition of the responses to noxious stimuli of SCT, STT and SMT neurones, whilst the responses to non-nociceptive stimuli, spontaneous activity and DLH-evoked activity were unaffected. This action was reversed in the presence of sulpiride, the highly selective D2 DA receptor antagonist. Neither sulpiride alone nor SKF38393, a D1 DA receptor agonist, altered evoked or spontaneous activity when ionophoretically applied. 3. Focal electrical stimulation in the region of the A11, but not the A9, DA cell group selectively suppressed nociceptive responses of spinal, multireceptive neurones in the rat. This stimulus-evoked effect was consistently and rapidly reversed by ionophoresis of sulpiride, in the vicinity of the dorsal horn neurone being tested. In contrast, naloxone and idazoxan (RX781094), an alpha 2-antagonist, were not effective. 4. This study presents data supporting a selective antinociceptive role for DA at the spinal level, where it has a widespread antinociceptive influence, on cells in both the superficial and deeper dorsal horn. The A11 DA cell group was shown to be a supraspinal site from which a selective antinociceptive action could be electrically evoked and which was mediated by DA at the level of the dorsal horn.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barasi S., Roberts M. T. Responses of motoneurones to electrophoretically applied dopamine. Br J Pharmacol. 1977 May;60(1):29–34. doi: 10.1111/j.1476-5381.1977.tb16743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Nobin A. Fluorescence histochemical and microspectrofluorometric mapping of dopamine and noradrenaline cell groups in the rat diencephalon. Brain Res. 1973 Mar 15;51:193–205. doi: 10.1016/0006-8993(73)90372-7. [DOI] [PubMed] [Google Scholar]
- Brown A. G., House C. R., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones in the cat. J Physiol. 1976 Sep;260(3):719–738. doi: 10.1113/jphysiol.1976.sp011540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. The spinocervical tract. Prog Neurobiol. 1981;17(1-2):59–96. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol. 1977 May;267(2):537–558. doi: 10.1113/jphysiol.1977.sp011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissiong J. W., Gentleman S., Neff N. H. Spinal cord dopaminergic neurons: evidence for an uncrossed nigrospinal pathway. Neuropharmacology. 1979 Jun;18(6):565–568. doi: 10.1016/0028-3908(79)90102-3. [DOI] [PubMed] [Google Scholar]
- Demenge P., Feuerstein C., Mouchet P., Guerin B. Stereospecific binding of 3H-haloperidol in rat dorsal spinal cord. Eur J Pharmacol. 1980 Aug 22;66(1):117–120. doi: 10.1016/0014-2999(80)90302-7. [DOI] [PubMed] [Google Scholar]
- Duggal K. N., Barasi S. Electrical stimulation of the substantia nigra does not increase tail-flick latency in the rat. Exp Neurol. 1985 Feb;87(2):300–308. doi: 10.1016/0014-4886(85)90220-1. [DOI] [PubMed] [Google Scholar]
- Euvrard C., Ferland L., Di Paolo T., Beaulieu M., Labrie F., Oberlander C., Raynaud J. P., Boissier J. R. Activity of two new potent dopaminergic agonists at the striatal and anterior pituitary levels. Neuropharmacology. 1980 Apr;19(4):379–386. doi: 10.1016/0028-3908(80)90190-2. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker S. M., Coote J. H. The contribution of brain stem catecholamine cell groups to the innervation of the sympathetic lateral cell column. Brain Res. 1981 Jan 26;205(1):141–155. doi: 10.1016/0006-8993(81)90726-5. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker S. M., Mitchell R., Hope P. J., Molony V., Iggo A. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res. 1985 May 20;334(2):243–254. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Hosoya Y. The distribution of spinal projection neurons in the hypothalamus of the rat, studied with the HRP method. Exp Brain Res. 1980;40(1):79–87. doi: 10.1007/BF00236665. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Phillipson O., Goldstein M. Evidence for a dopaminergic pathway in the rat descending from the A11 cell group to the spinal cord. Acta Physiol Scand. 1979 Dec;107(4):393–395. doi: 10.1111/j.1748-1716.1979.tb06491.x. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. M., Palkovits M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. I. Forebrain (telencephalon, diencephalon). J Comp Neurol. 1974 Sep 1;157(1):13–28. doi: 10.1002/cne.901570103. [DOI] [PubMed] [Google Scholar]
- Jensen T. S., Schrøder H. D., Smith D. F. The role of spinal pathways in dopamine mediated alteration in the tail-flick reflex in rats. Neuropharmacology. 1984 Feb;23(2A):149–153. doi: 10.1016/s0028-3908(84)80006-4. [DOI] [PubMed] [Google Scholar]
- Jensen T. S., Smith D. F. Role of 5-HT and NA in spinal dopaminergic analgesia. Eur J Pharmacol. 1982 Dec 17;86(1):65–70. doi: 10.1016/0014-2999(82)90397-1. [DOI] [PubMed] [Google Scholar]
- Jensen T. S., Smith D. F. Stimulation of spinal dopaminergic receptors: differential effects on tail reflexes in rats. Neuropharmacology. 1983 Apr;22(4):477–483. doi: 10.1016/0028-3908(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Jensen T. S., Yaksh T. L. Effects of an intrathecal dopamine agonist, apomorphine, on thermal and chemical evoked noxious responses in rats. Brain Res. 1984 Apr 2;296(2):285–293. doi: 10.1016/0006-8993(84)90064-7. [DOI] [PubMed] [Google Scholar]
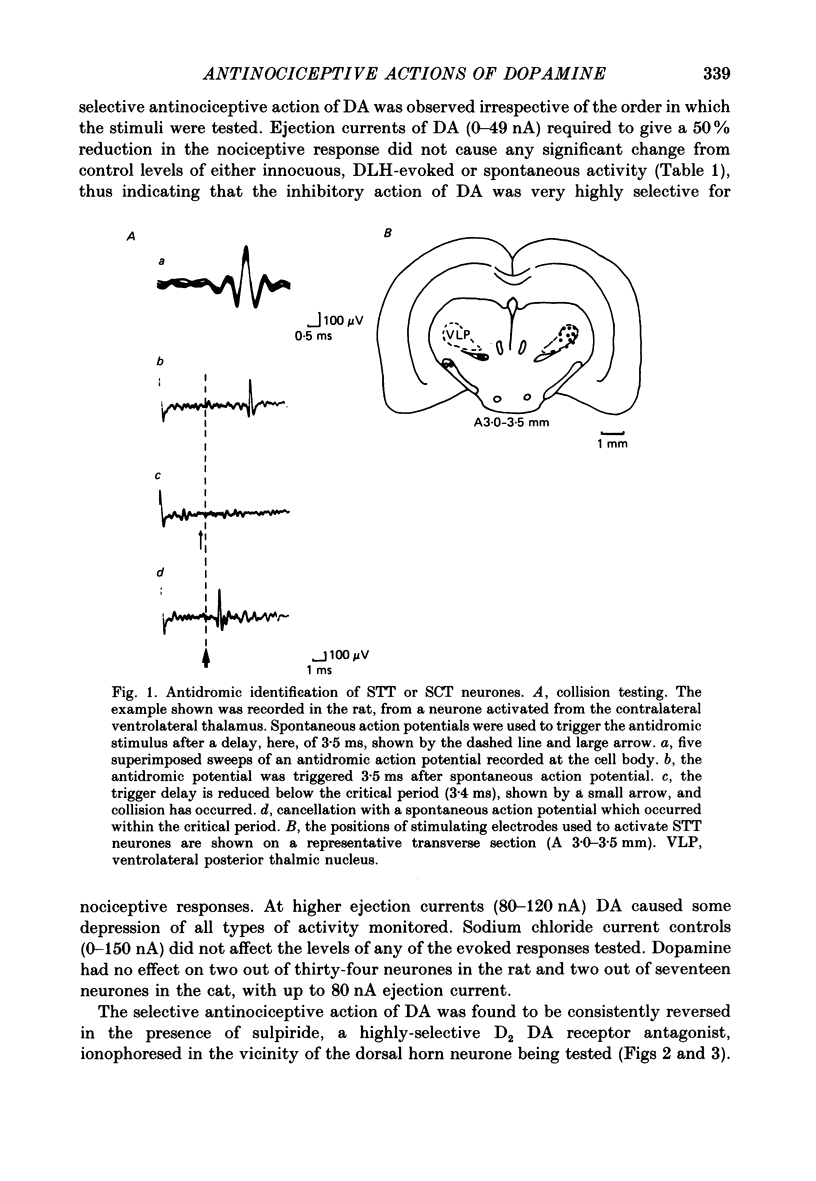
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981 Jun;4(1):1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Giesler G. J., Jr, Besson J. M. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977 Jan 18;27(1):15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Scatton B., Dubois A., Cudennec A. Autoradiographic localization of dopamine receptors in the spinal cord of the rat using [3H]-N-propylnorapomorphine. J Neural Transm. 1984;59(3):251–256. doi: 10.1007/BF01250012. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Setler P. E., Sarau H. M., Zirkle C. L., Saunders H. L. The central effects of a novel dopamine agonist. Eur J Pharmacol. 1978 Aug 15;50(4):419–430. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- Skagerberg G., Björklund A., Lindvall O., Schmidt R. H. Origin and termination of the diencephalo-spinal dopamine system in the rat. Brain Res Bull. 1982 Jul-Dec;9(1-6):237–244. doi: 10.1016/0361-9230(82)90136-8. [DOI] [PubMed] [Google Scholar]
- Skagerberg G., Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985 Sep 9;342(2):340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]


