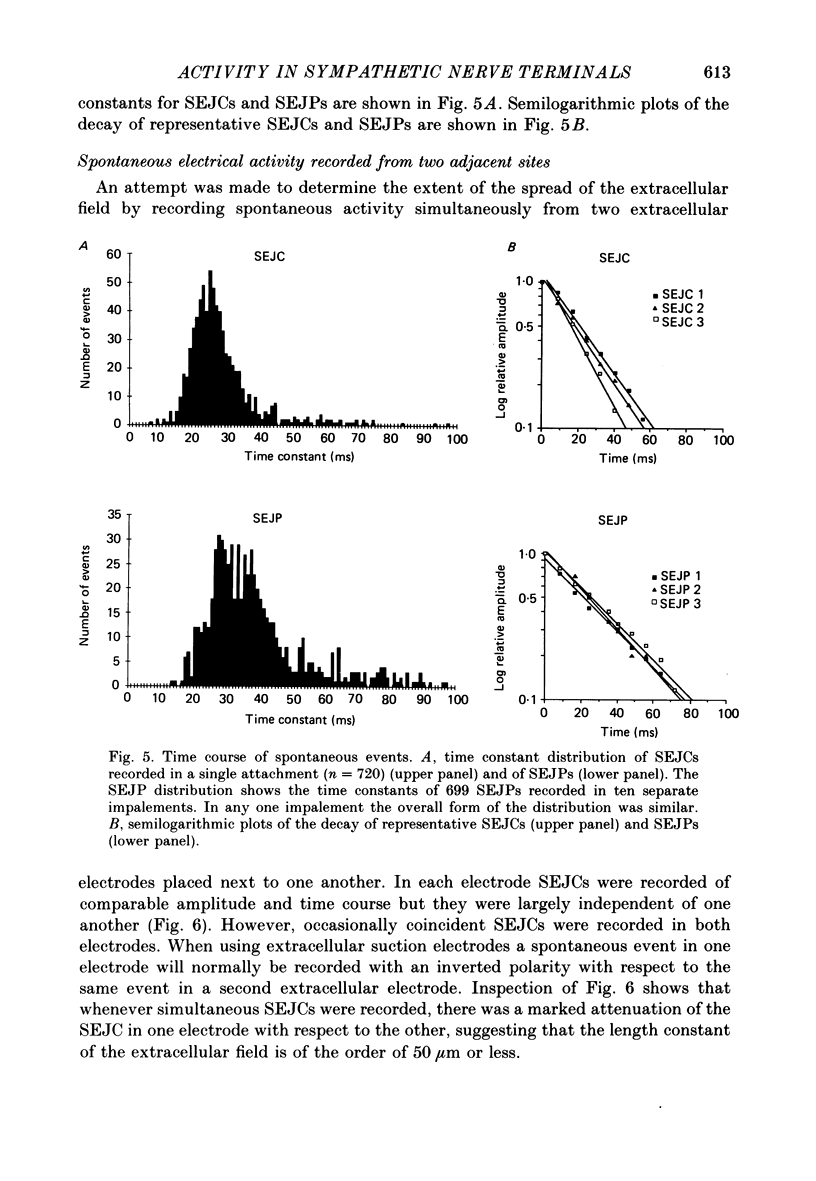
Abstract
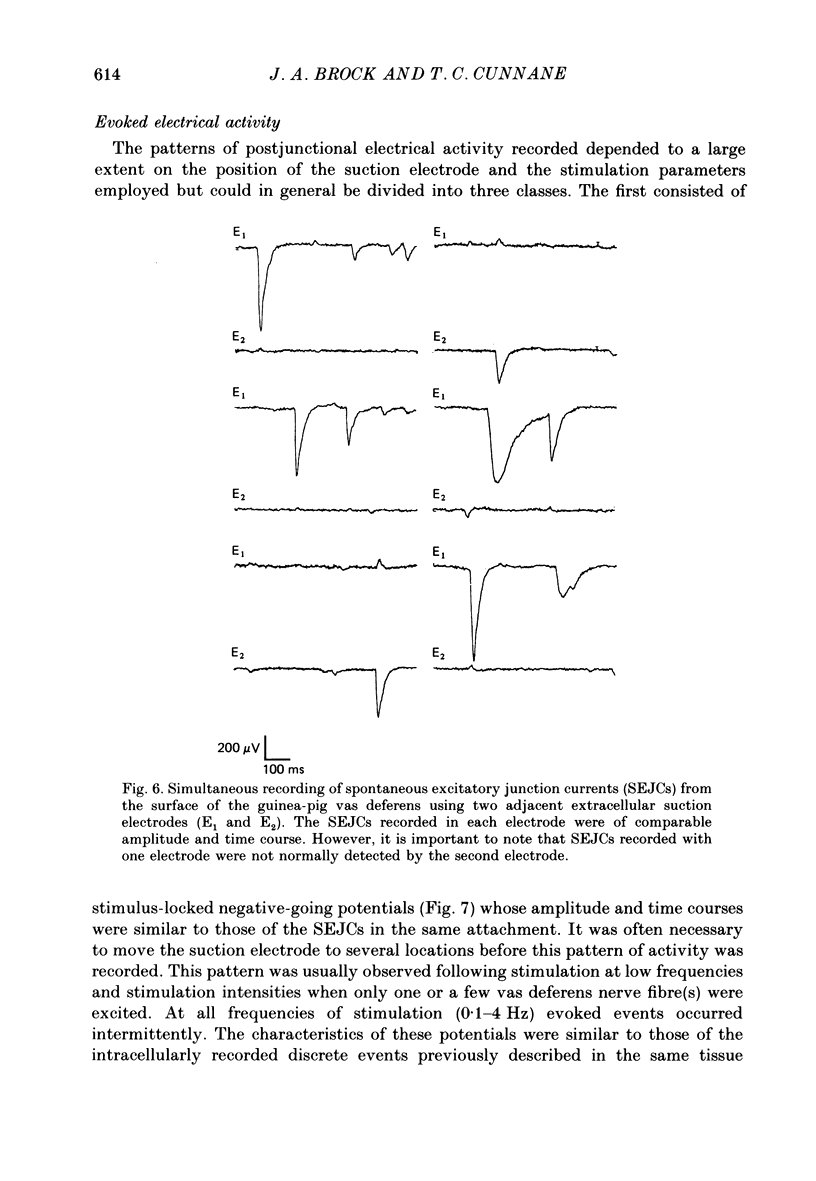
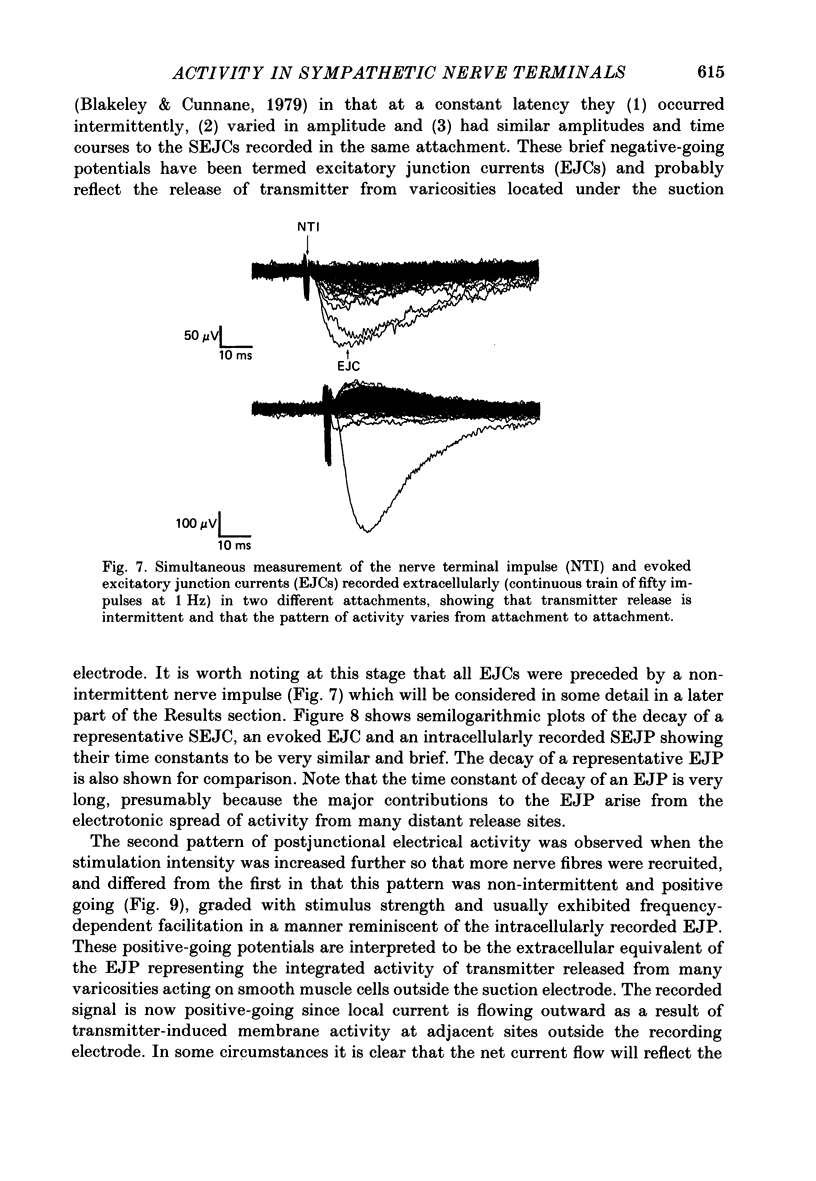
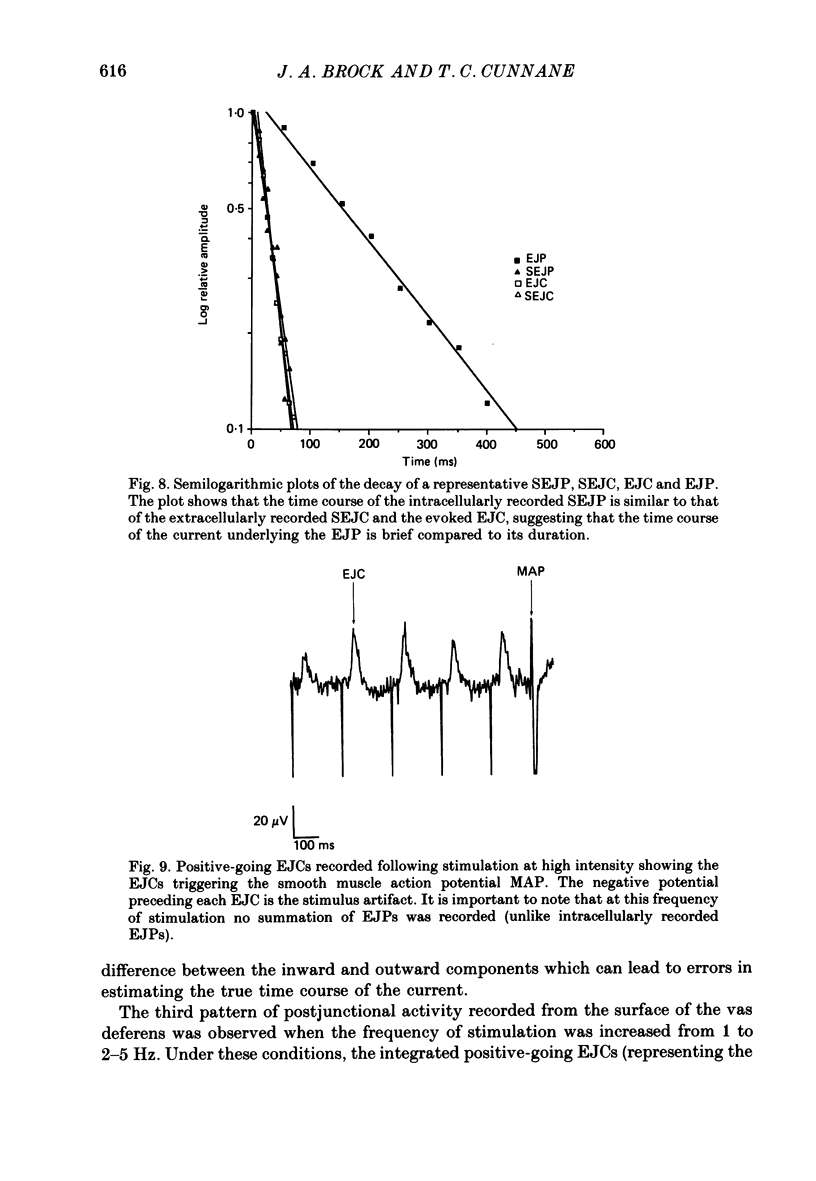
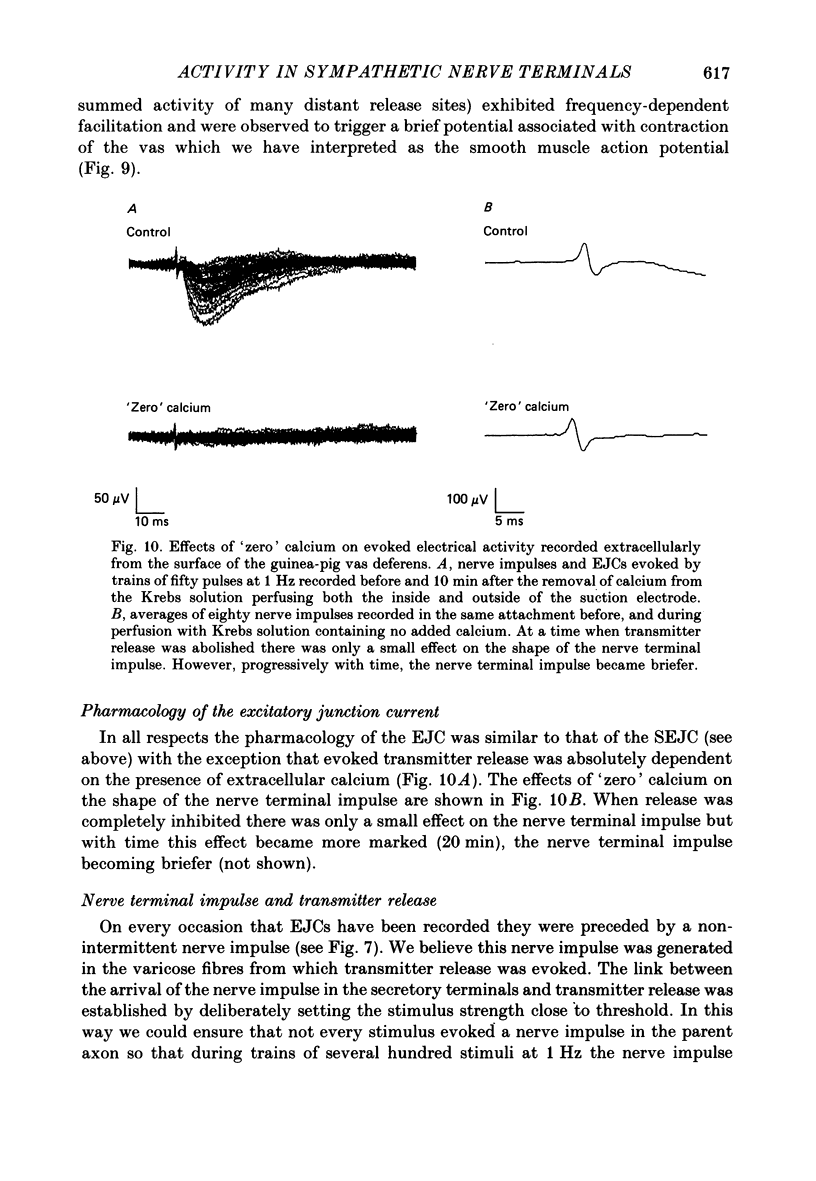
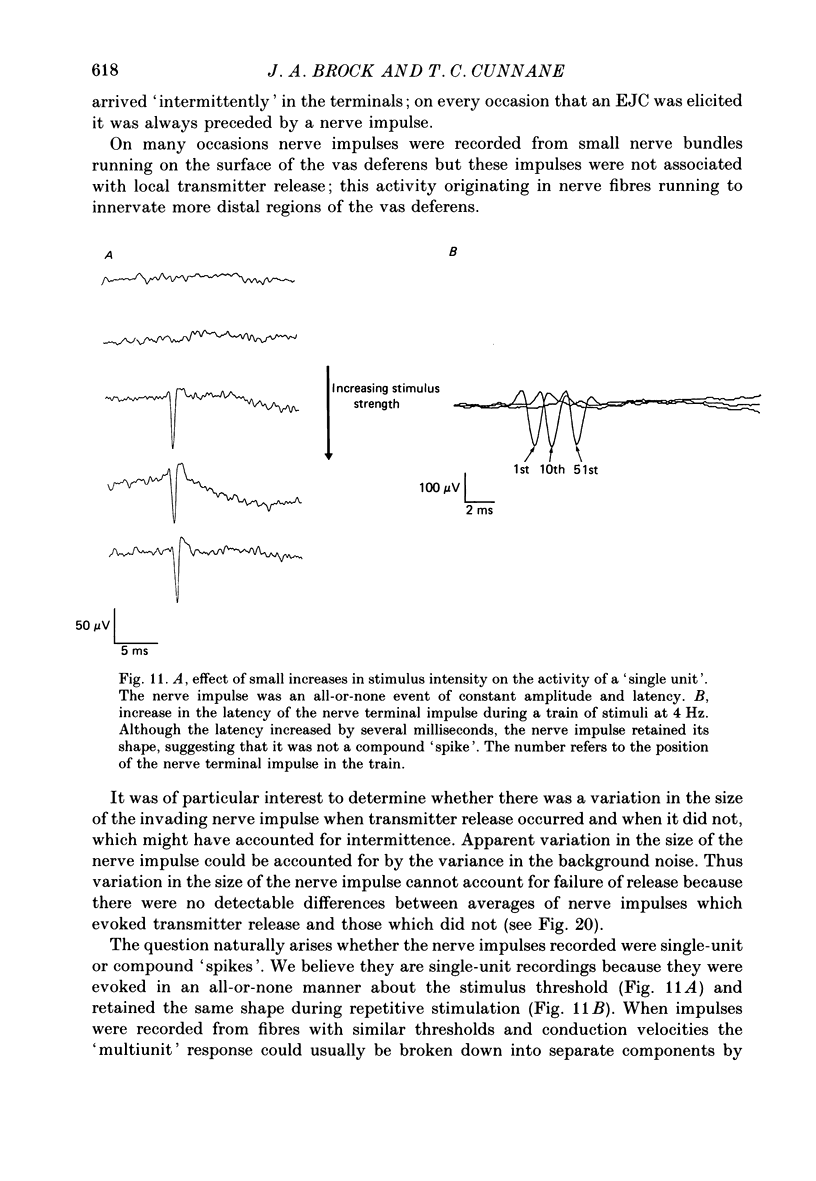
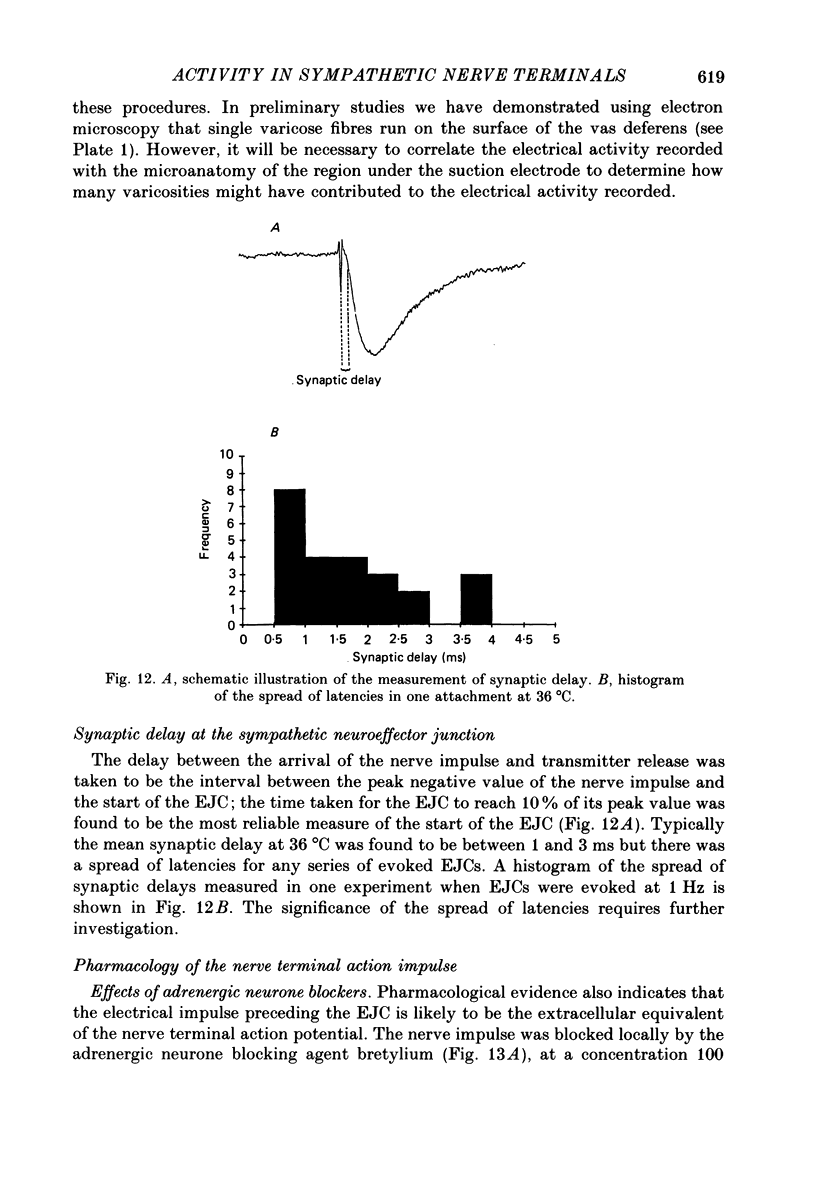
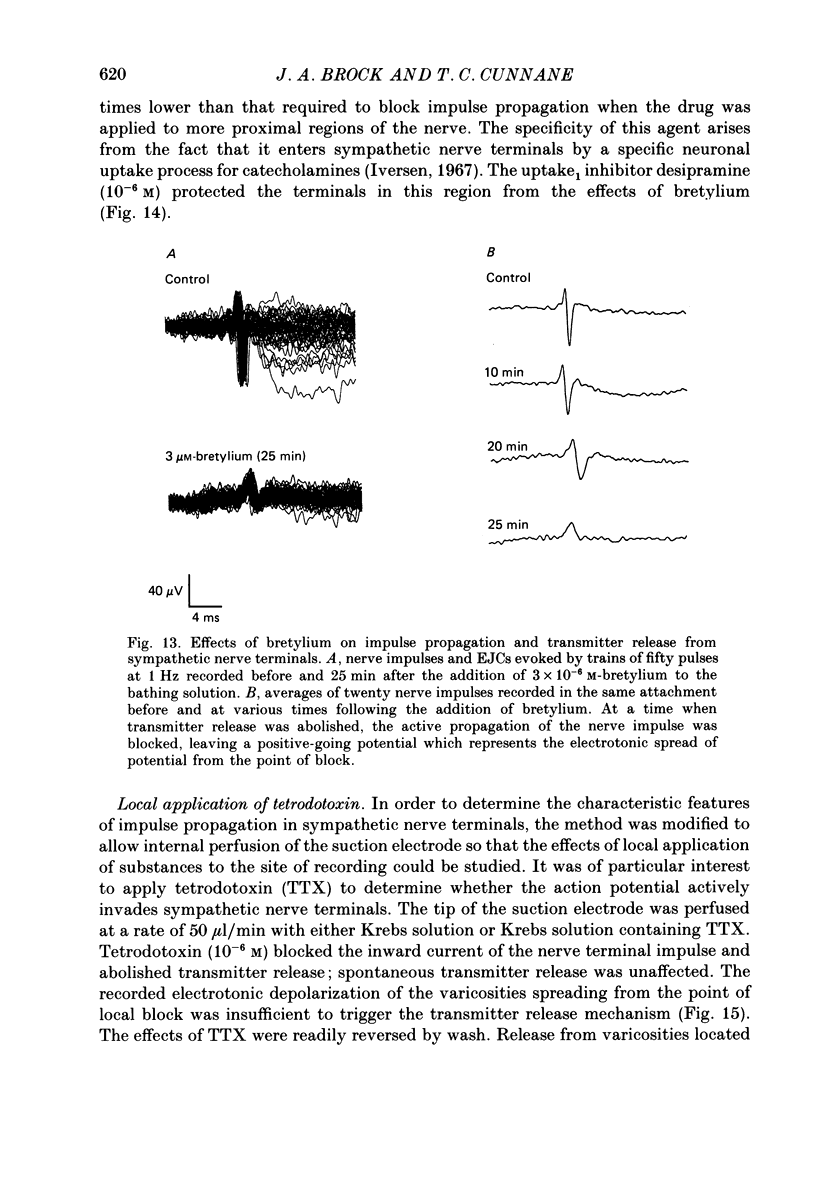
1. The relationship between the nerve terminal action potential and transmitter release from sympathetic postganglionic nerve terminals has been studied in vitro by focal extracellular recording. 2. In the absence of stimulation, 'spontaneous excitatory junction currents' (SEJCs) were recorded with amplitudes up to 500 microV, durations of 50-80 ms and frequencies of occurrence of 0.3-0.05 Hz; SEJCs of unusually long time course were also observed. The SEJCs were not recorded in tissues pre-treated with 6-hydroxydopamine to destroy sympathetic nerves, were unaffected by tetrodotoxin (TTX), the competitive alpha-adrenoceptor antagonists, prazosin and phentolamine, the irreversible alpha-adrenoceptor antagonist benextramine but were blocked by alpha,beta-methylene ATP which desensitizes P2-purinoceptors. 3. During trains of supramaximal stimuli at 0.1-4 Hz stimulus locked 'excitatory junction currents' (EJCs) were evoked intermittently from the population of varicosities located under the suction electrode with a probability of occurrence of 0.005-0.8. Although EJCs occurred intermittently, they were always preceded by an associated, non-intermittent, nerve impulse (delay less than or equal to 3 ms). 4. The EJCs reflect transmitter release from nerves because they were abolished by TTX, removal of calcium from the bathing medium, exposure to alpha-beta-methylene ATP and exhibited frequency-dependent facilitation. 5. Amplitude distributions of SEJCs and EJCs recorded in the same attachment were similar and skewed towards low-amplitude events. Individual SEJCs and EJCs could be found which were identical in amplitude and time course. 6. Locally applied TTX blocked impulse propagation and transmitter release in the terminal region; electrotonic invasion of the terminals from the point of block did not activate the transmitter release process. 7. These studies indicate that (1) intermittence of transmitter release is caused by a low probability of release in the invaded varicosity and is not caused by conduction failure in the terminal regions, (2) only a single quantum is normally secreted when the release mechanism of a varicosity is activated by the nerve impulse and (3) active invasion of the terminals is necessary for transmitter release to occur.
Full text
PDF


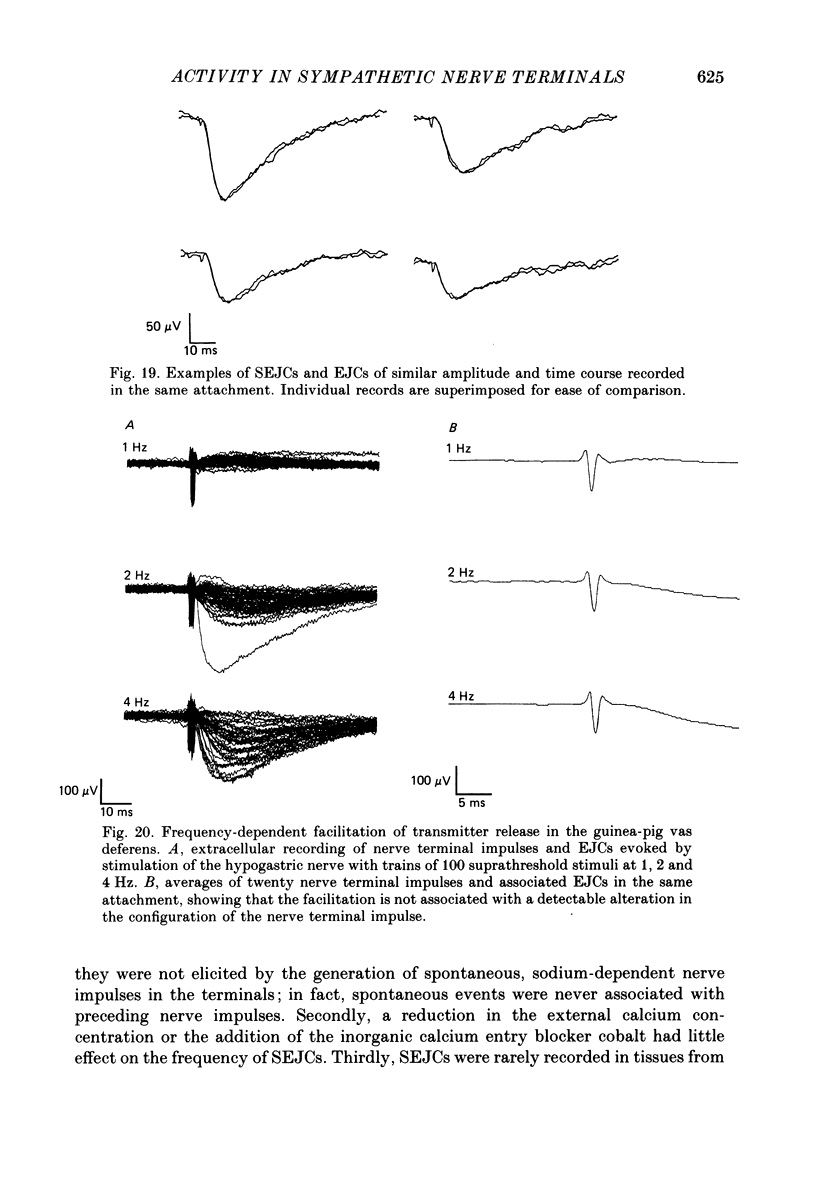
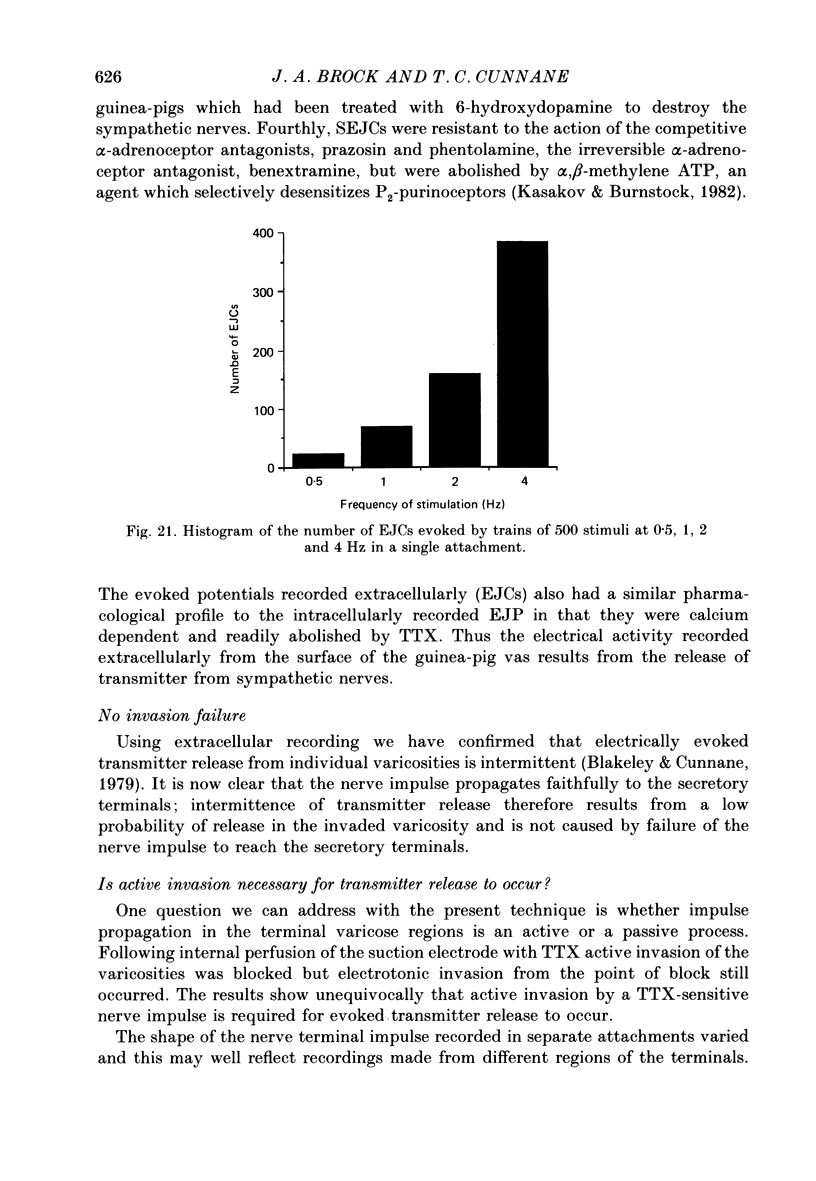






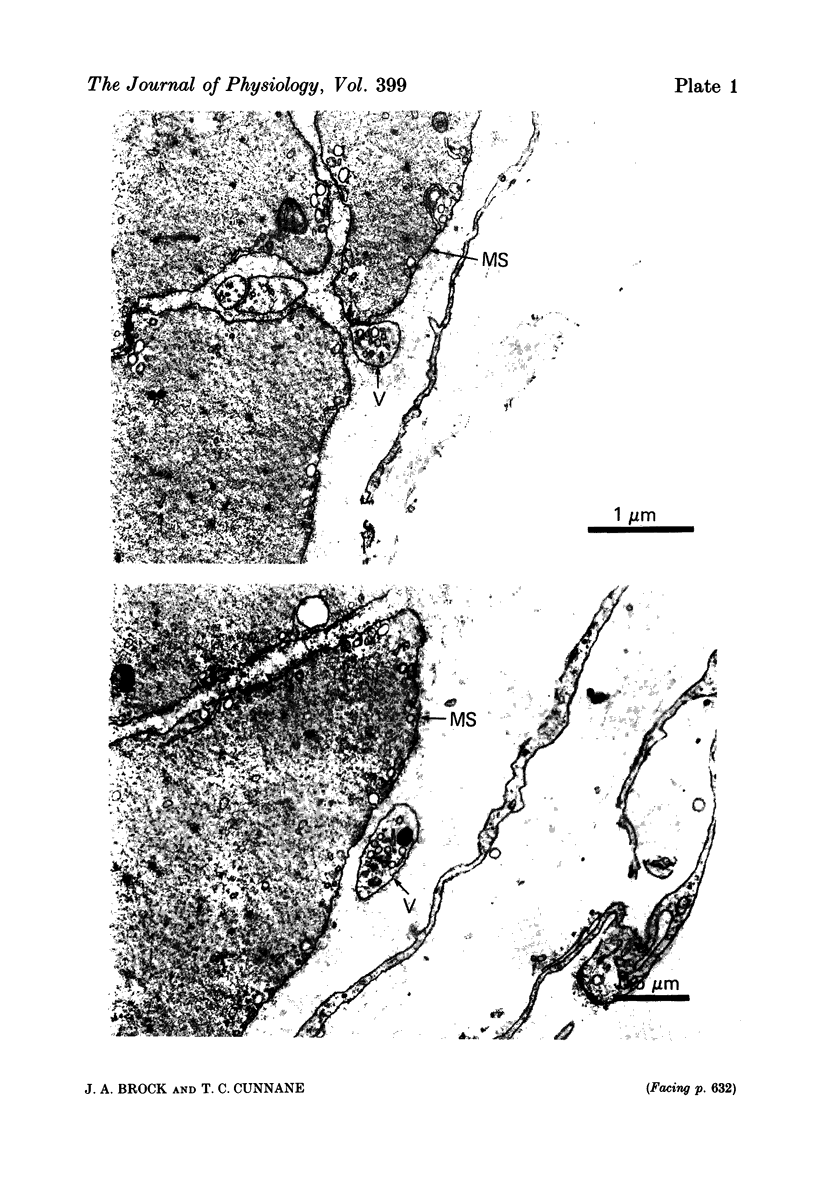
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts P., Bartfai T., Stjärne L. Site(s) and ionic basis of alpha-autoinhibition and facilitation of "3H'noradrenaline secretion in guinea-pig vas deferens. J Physiol. 1981 Mar;312:297–334. doi: 10.1113/jphysiol.1981.sp013630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allcorn R. J., Cunnane T. C., Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986 Dec;89(4):647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Spontaneous potential at sympathetic nerve endings in smooth muscle. J Physiol. 1962 Mar;160:446–460. doi: 10.1113/jphysiol.1962.sp006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Chesher G. B., Su C. Release of adrenergic transmitter from terminal nerve plexus in artery. Agents Actions. 1969 Jul;1(1):20–26. doi: 10.1007/BF01990016. [DOI] [PubMed] [Google Scholar]
- Blakeley A. G., Cunnane T. C. The packeted release of transmitter from the sympathetic nerves of the guinea-pig vas deferens: an electrophysiological study. J Physiol. 1979 Nov;296:85–96. doi: 10.1113/jphysiol.1979.sp012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature. 1987 Apr 9;326(6113):605–607. doi: 10.1038/326605a0. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane T. C., Stjärne L. Frequency dependent intermittency and ionic basis of impulse conduction in postganglionic sympathetic fibres of guinea-pig vas deferens. Neuroscience. 1984 Jan;11(1):211–229. doi: 10.1016/0306-4522(84)90225-2. [DOI] [PubMed] [Google Scholar]
- Cunnane T. C., Stjärne L. Transmitter secretion from individual varicosities of guinea-pig and mouse vas deferens: highly intermittent and monoquantal. Neuroscience. 1984 Sep;13(1):1–20. doi: 10.1016/0306-4522(84)90255-0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The histological localization of noradrenaline in the cat spleen. J Physiol. 1966 Nov;187(1):69–79. doi: 10.1113/jphysiol.1966.sp008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand. 1974 Nov;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P. Non-uniform release at the frog neuromuscular junction: evidence of morphological and physiological plasticity. Brain Res. 1987 Mar;434(1):95–116. doi: 10.1016/0165-0173(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Discrete events measure single quanta of adenosine 5'-triphosphate secreted from sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1984 Sep;13(1):21–28. doi: 10.1016/0306-4522(84)90256-2. [DOI] [PubMed] [Google Scholar]
- Totterdell S., Smith A. D. Cholecystokinin-immunoreactive boutons in synaptic contact with hippocampal pyramidal neurons that project to the nucleus accumbens. Neuroscience. 1986 Sep;19(1):181–192. doi: 10.1016/0306-4522(86)90014-x. [DOI] [PubMed] [Google Scholar]