Abstract
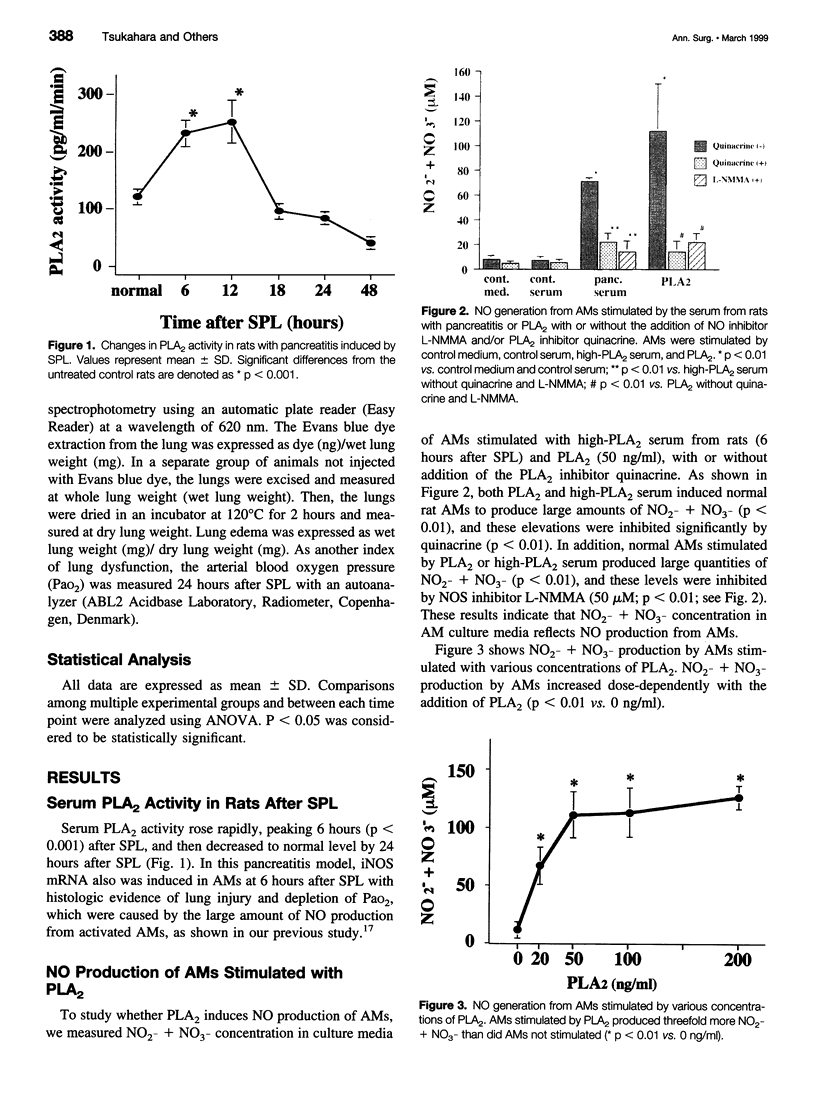
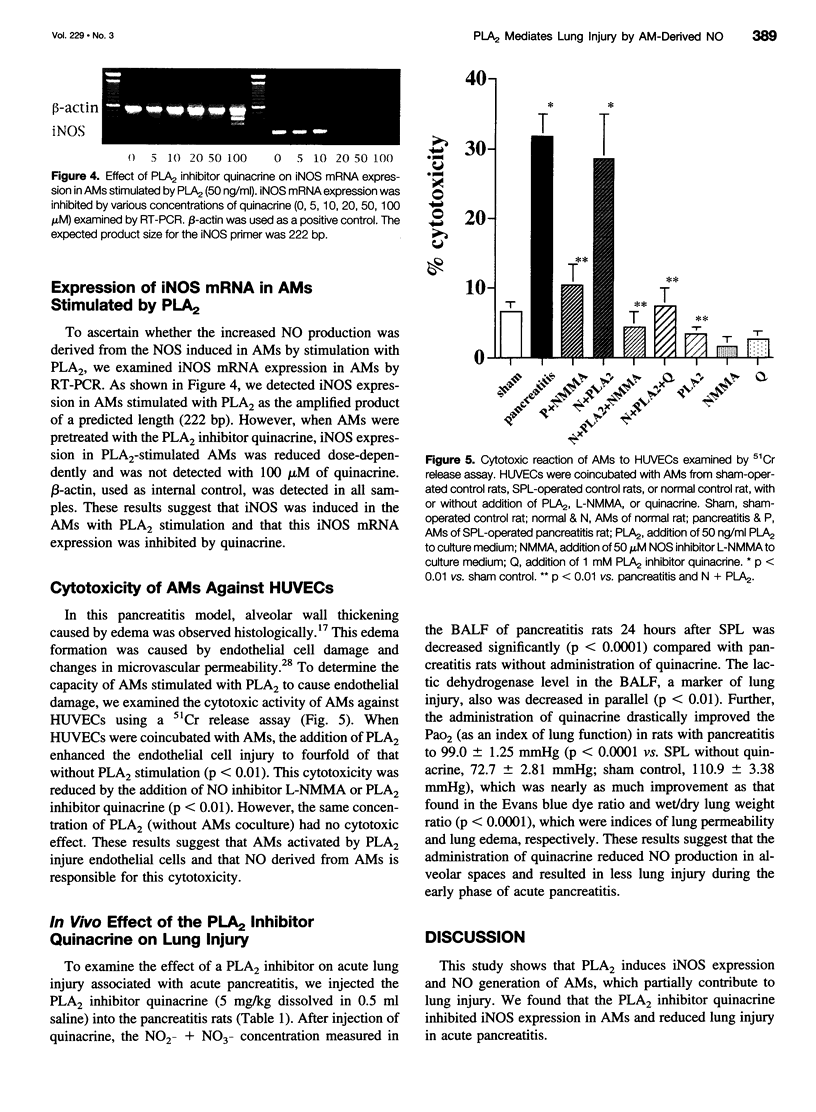
OBJECTIVE: Reportedly, nitric oxide (NO) derived from alveolar macrophages (AMs) and increased serum phospholipase A2 (PLA2) activity are associated with the pathogenesis of lung injury in acute pancreatitis. The authors examined the possibility that PLA2 causes, in part, the induction of NO production by AMs in pancreatitis. METHODS: Pancreatitis was induced in rats by selective pancreatic duct ligation (SPL). AMs were stimulated with PLA2 or SPL rat serum, with or without administration of the PLA2 inhibitor quinacrine. Then NO production from the AMs was measured by the Griess method, inducible NO synthase mRNA expression of AMs was analyzed by the reverse transcription-polymerase chain reaction, and cytotoxic effects of AMs on human umbilical vein endothelial cells was examined by a 51Cr release assay. In vivo, the effect of quinacrine on lung injury was determined by measuring the arterial blood oxygen pressure (PaO2), lung weight, and lung permeability using Evans blue dye concentration of SPL rat. RESULTS: In vitro, the serum with high PLA2 activity induced NO production by rat AMs. PLA2 (50 ng/ml) induced significant amounts of NO production, inducible NO synthase mRNA expression, and cytotoxicity toward the human umbilical vein endothelial cells in normal rat AMs, and these activities were significantly inhibited by quinacrine. In vivo, rats with pancreatitis that were given quinacrine showed decreased concentrations of NO2- and NO3- in the bronchoalveolar lavage fluid, and the PaO2, lung edema, and lung permeability were improved significantly. CONCLUSION: PLA2 induces AMs to release NO, which contributes to lung injury in acute pancreatitis. This lung injury was prevented by the administration of the PLA2 inhibitor quinacrine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. O., Moore E. E., Banerjee A. Phospholipase A2 regulates critical inflammatory mediators of multiple organ failure. J Surg Res. 1994 Feb;56(2):199–205. doi: 10.1006/jsre.1994.1032. [DOI] [PubMed] [Google Scholar]
- Bomalaski J. S., Ford T., Hudson A. P., Clark M. A. Phospholipase A2-activating protein induces the synthesis of IL-1 and TNF in human monocytes. J Immunol. 1995 Apr 15;154(8):4027–4031. [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Schädlich H., Nevalainen T. J., Friess H., Beger H. G. Role of phospholipase A2 in human acute pancreatitis. Gastroenterology. 1989 Dec;97(6):1521–1526. doi: 10.1016/0016-5085(89)90398-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Closa D., Bardají M., Hotter G., Prats N., Gelpí E., Fernández-Cruz L., Roselló-Catafau J. Hepatic involvement in pancreatitis-induced lung damage. Am J Physiol. 1996 Jan;270(1 Pt 1):G6–13. doi: 10.1152/ajpgi.1996.270.1.G6. [DOI] [PubMed] [Google Scholar]
- Dana R., Malech H. L., Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994 Jan 1;297(Pt 1):217–223. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana R., Malech H. L., Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994 Jan 1;297(Pt 1):217–223. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Scott M. T., McCuiston S. Effects of experimental acute pancreatitis in dogs on metabolism of lung surfactant phosphatidylcholine. Biochem Biophys Res Commun. 1987 May 29;145(1):612–618. doi: 10.1016/0006-291x(87)91364-7. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Edelson J. D., Vadas P., Villar J., Mullen J. B., Pruzanski W. Acute lung injury induced by phospholipase A2. Structural and functional changes. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1102–1109. doi: 10.1164/ajrccm/143.5_Pt_1.1102. [DOI] [PubMed] [Google Scholar]
- Formela L. J., Galloway S. W., Kingsnorth A. N. Inflammatory mediators in acute pancreatitis. Br J Surg. 1995 Jan;82(1):6–13. doi: 10.1002/bjs.1800820105. [DOI] [PubMed] [Google Scholar]
- Gukovskaya A. S., Perkins P., Zaninovic V., Sandoval D., Rutherford R., Fitzsimmons T., Pandol S. J., Poucell-Hatton S. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996 Mar;110(3):875–884. doi: 10.1053/gast.1996.v110.pm8608898. [DOI] [PubMed] [Google Scholar]
- Gunasekar P. G., Kanthasamy A. G., Borowitz J. L., Isom G. E. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem. 1995 Nov;65(5):2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- Kurose I., Saito H., Miura S., Ebinuma H., Higuchi H., Watanabe N., Zeki S., Nakamura T., Takaishi M., Ishii H. CD18/ICAM-1-dependent oxidative NF-kappaB activation leading to nitric oxide production in rat Kupffer cells cocultured with syngeneic hepatoma cells. J Clin Invest. 1997 Mar 1;99(5):867–878. doi: 10.1172/JCI119251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechin A. E., Varon J. Adult respiratory distress syndrome (ARDS): the basics. J Emerg Med. 1994 Jan-Feb;12(1):63–68. doi: 10.1016/0736-4679(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Markewitz B. A., Michael J. R., Kohan D. E. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest. 1993 May;91(5):2138–2143. doi: 10.1172/JCI116439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki T., Goya T., Toh H., Nishihara K., Torisu M. The anti Mac-1 monoclonal antibody inhibits neutrophil sequestration in lung and liver in a septic murine model. Clin Immunol Immunopathol. 1991 Dec;61(3):365–375. doi: 10.1016/s0090-1229(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Mäkelä A., Kuusi T., Schröder T. Serum phospholipase A2, amylase, lipase, and urinary amylase activities in relation to the severity of acute pancreatitis. Eur J Surg. 1997 Dec;163(12):915–922. [PubMed] [Google Scholar]
- Neilly I. J., Copland M., Haj M., Adey G., Benjamin N., Bennett B. Plasma nitrate concentrations in neutropenic and non-neutropenic patients with suspected septicaemia. Br J Haematol. 1995 Jan;89(1):199–202. doi: 10.1111/j.1365-2141.1995.tb08931.x. [DOI] [PubMed] [Google Scholar]
- Nevalainen T. J., Grönroos J. M., Kortesuo P. T. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut. 1993 Aug;34(8):1133–1136. doi: 10.1136/gut.34.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T. J. Serum phospholipases A2 in inflammatory diseases. Clin Chem. 1993 Dec;39(12):2453–2459. [PubMed] [Google Scholar]
- Nordback I., Teerenhovi O., Auvinen O., Koivula T., Thuren T., Kinnunen P., Eskola J., Näntö V. Human pancreatic phospholipase A2 in acute necrotizing pancreatitis. Digestion. 1989;42(3):128–134. doi: 10.1159/000199837. [DOI] [PubMed] [Google Scholar]
- Nunokawa Y., Ishida N., Tanaka S. Cloning of inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Feb 26;191(1):89–94. doi: 10.1006/bbrc.1993.1188. [DOI] [PubMed] [Google Scholar]
- Nyman K. M., Uhl W., Forsström J., Büchler M., Beger H. G., Nevalainen T. J. Serum phospholipase A2 in patients with multiple organ failure. J Surg Res. 1996 Jan;60(1):7–14. doi: 10.1006/jsre.1996.0003. [DOI] [PubMed] [Google Scholar]
- Offenstadt G., Pinta P., Masliah J., Alcindor L. G., Héricord P., Amstutz P. Phospholipase and prophospholipase activities in bronchoalveolar lavage fluid in severe acute pulmonary disease with or without ARDS. Intensive Care Med. 1981;7(6):285–290. doi: 10.1007/BF01709723. [DOI] [PubMed] [Google Scholar]
- Patterson C. E., Rhoades R. A., Garcia J. G. Evans blue dye as a marker of albumin clearance in cultured endothelial monolayer and isolated lung. J Appl Physiol (1985) 1992 Mar;72(3):865–873. doi: 10.1152/jappl.1992.72.3.865. [DOI] [PubMed] [Google Scholar]
- Payá M., García Pastor P., Coloma J., Alcaraz M. J. Nitric oxide synthase and cyclo-oxygenase pathways in the inflammatory response induced by zymosan in the rat air pouch. Br J Pharmacol. 1997 Apr;120(8):1445–1452. doi: 10.1038/sj.bjp.0701073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner I. G., Savage W. T., 3rd, Pantoja J. L., Renner V. J. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985 Oct;30(10):1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- Rogers D. F., Boschetto P., Barnes P. J. Plasma exudation. Correlation between Evans blue dye and radiolabeled albumin in guinea pig airways in vivo. J Pharmacol Methods. 1989 Jul;21(4):309–315. doi: 10.1016/0160-5402(89)90068-5. [DOI] [PubMed] [Google Scholar]
- Sano M., Morishita T., Nozaki M., Yokoyama M., Watanabe Y., Nakano H. Elevation of the phospholipase A2 activity in peritoneal fluid cells from women with endometriosis. Fertil Steril. 1994 Apr;61(4):657–662. doi: 10.1016/s0015-0282(16)56642-4. [DOI] [PubMed] [Google Scholar]
- Santos A. A., Browning J. L., Scheltinga M. R., Lynch E. A., Brown E. F., Lawton P., Chambers E., Dougas I., Benjamin C. D., Dinarello C. A. Are events after endotoxemia related to circulating phospholipase A2? Ann Surg. 1994 Feb;219(2):183–192. doi: 10.1097/00000658-199402000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Tsukahara Y., Horita Y., Anan K., Morisaki T., Tanaka M., Torisu M. Role of nitric oxide derived from alveolar macrophages in the early phase of acute pancreatitis. J Surg Res. 1996 Nov;66(1):43–50. doi: 10.1006/jsre.1996.0370. [DOI] [PubMed] [Google Scholar]
- Uchiyama A., Morisaki T., Torisu M. Evidence that induction and regulation of lymphokine-activated killer (LAK) activity are mediated by changes in tumour-binding potential of lymphocytes after activation by interleukin-2 (IL-2). Immunology. 1991 Sep;74(1):94–98. [PMC free article] [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Induction of group II phospholipase A2 expression and pathogenesis of the sepsis syndrome. Circ Shock. 1993 Feb;39(2):160–167. [PubMed] [Google Scholar]
- Weiss J., Inada M., Elsbach P., Crowl R. M. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994 Oct 21;269(42):26331–26337. [PubMed] [Google Scholar]
- Werner J., Rivera J., Fernandez-del Castillo C., Lewandrowski K., Adrie C., Rattner D. W., Warshaw A. L. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery. 1997 Jan;121(1):23–30. doi: 10.1016/s0039-6060(97)90178-1. [DOI] [PubMed] [Google Scholar]
- Zhou W., McCollum M. O., Levine B. A., Olson M. S. Role of platelet-activating factor in pancreatitis-associated acute lung injury in the rat. Am J Pathol. 1992 Apr;140(4):971–979. [PMC free article] [PubMed] [Google Scholar]



