Abstract
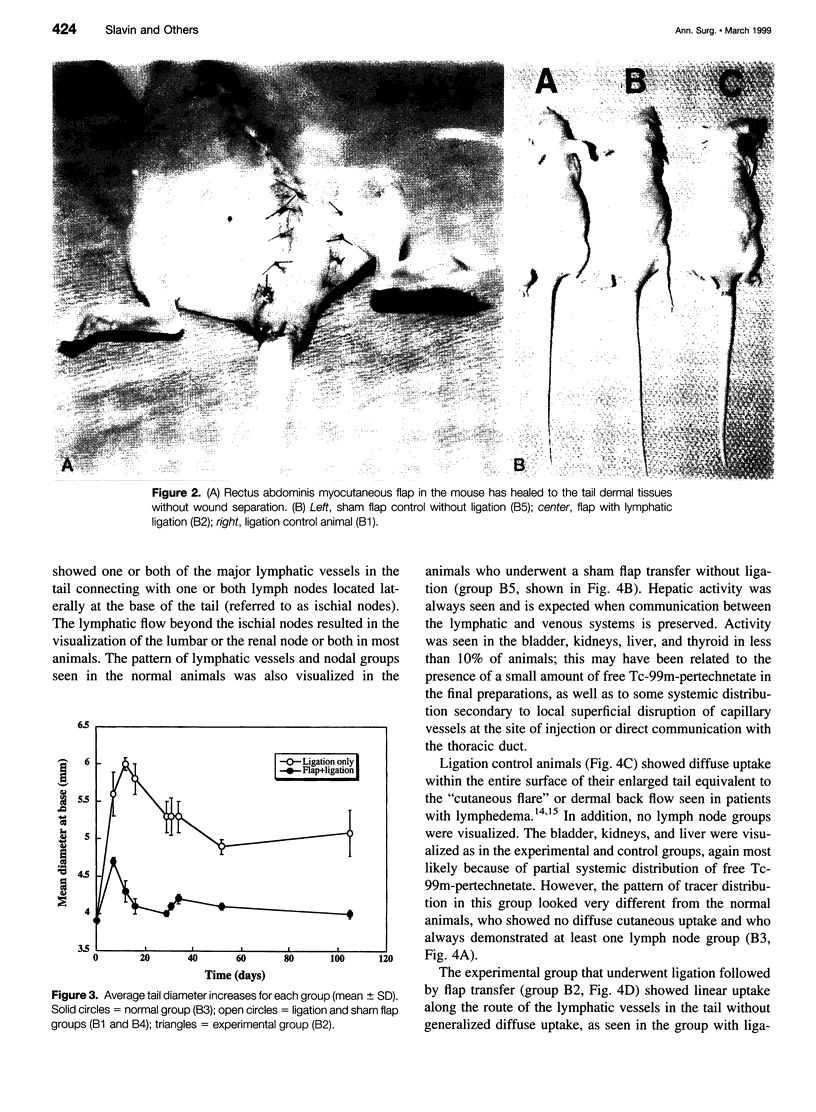
OBJECTIVE: The goals of this work were to develop animal models of lymphedema and tissue flap transfer, and to observe physiologic changes in lymphatic function that occur in these models over time, both systemically with lymphoscintigraphy (LS) and locally using fluorescence microlymphangiography (FM). SUMMARY BACKGROUND DATA: Although lymphedema has been managed by a combination of medical and surgical approaches, no effective long-term cure exists. Surgical attempts aimed at reconnecting impaired lymphatic channels or bypassing obstructed areas have failed. METHODS: The tails of rats (A groups) and mice (B groups) were used because of their different features. Lymphedema was created by ligation of the lymphatics at the tail base and quantified by diameter measurements there. In the experimental group, rectus abdominis myocutaneous flap was transferred across the ligation. In addition to the ligation (A1 and B1) and ligation + flap (A2 and B2) groups, three control groups were included: sham flap with ligation (B4), sham flap alone (B5), and normal (A3 and B3) animals. Observations were made at weekly time points for lymphatic function and continuity. RESULTS: Lymphedema was successfully created in the mouse ligation groups (B1 and B4) and sustained for the entire length of observation (up to 14 weeks). Lymphatic continuity was restored in those animals with transferred flaps across the ligation site (A2 and B2), as seen both by LS and FM. Sham flaps did not visibly affect lymphatic function nor did they cause any visible swelling in the tail. CONCLUSIONS: Acute lymphedema developing after ligation of tail lymphatics in mice can be prevented by myocutaneous flap transfer. Restored lymphatic continuity and function were demonstrable using lymphoscintigraphy and fluorescence microlymphangiography.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister R. G., Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990 Jan;85(1):64–76. doi: 10.1097/00006534-199001000-00012. [DOI] [PubMed] [Google Scholar]
- Bollinger A. Microlymphatics of human skin. Int J Microcirc Clin Exp. 1993 Feb;12(1):1–15. [PubMed] [Google Scholar]
- Campisi C., Boccardo F., Alitta P., Tacchella M. Derivative lymphatic microsurgery: indications, techniques, and results. Microsurgery. 1995;16(7):463–468. doi: 10.1002/micr.1920160706. [DOI] [PubMed] [Google Scholar]
- Huang G. K., Hu R. Q., Liu Z. Z., Shen Y. L., Lan T. D., Pan G. P. Microlymphaticovenous anastomosis in the treatment of lower limb obstructive lymphedema: analysis of 91 cases. Plast Reconstr Surg. 1985 Nov;76(5):671–685. [PubMed] [Google Scholar]
- Ivens D., Hoe A. L., Podd T. J., Hamilton C. R., Taylor I., Royle G. T. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992 Jul;66(1):136–138. doi: 10.1038/bjc.1992.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter M. A., Slavin S. A., Kaplan W. An experimental model for chronic lymphedema. Plast Reconstr Surg. 1990 Apr;85(4):573–580. doi: 10.1097/00006534-199004000-00012. [DOI] [PubMed] [Google Scholar]
- Leu A. J., Berk D. A., Yuan F., Jain R. K. Flow velocity in the superficial lymphatic network of the mouse tail. Am J Physiol. 1994 Oct;267(4 Pt 2):H1507–H1513. doi: 10.1152/ajpheart.1994.267.4.H1507. [DOI] [PubMed] [Google Scholar]
- Mandell G. A., Alexander M. A., Harcke H. T. A multiscintigraphic approach to imaging of lymphedema and other causes of the congenitally enlarged extremity. Semin Nucl Med. 1993 Oct;23(4):334–346. doi: 10.1016/s0001-2998(05)80112-6. [DOI] [PubMed] [Google Scholar]
- McNeill G. C., Witte M. H., Witte C. L., Williams W. H., Hall J. N., Patton D. D., Pond G. D., Woolfenden J. M. Whole-body lymphangioscintigraphy: preferred method for initial assessment of the peripheral lymphatic system. Radiology. 1989 Aug;172(2):495–502. doi: 10.1148/radiology.172.2.2748831. [DOI] [PubMed] [Google Scholar]
- Mortimer P. S. Therapy approaches for lymphedema. Angiology. 1997 Jan;48(1):87–91. doi: 10.1177/000331979704800114. [DOI] [PubMed] [Google Scholar]
- Pezner R. D., Patterson M. P., Hill L. R., Lipsett J. A., Desai K. R., Vora N., Wong J. Y., Luk K. H. Arm lymphedema in patients treated conservatively for breast cancer: relationship to patient age and axillary node dissection technique. Int J Radiat Oncol Biol Phys. 1986 Dec;12(12):2079–2083. doi: 10.1016/0360-3016(86)90005-2. [DOI] [PubMed] [Google Scholar]
- Savage R. C. The surgical management of lymphedema. Surg Gynecol Obstet. 1984 Nov;159(5):501–508. [PubMed] [Google Scholar]
- Slavin S. A., Upton J., Kaplan W. D., Van den Abbeele A. D. An investigation of lymphatic function following free-tissue transfer. Plast Reconstr Surg. 1997 Mar;99(3):730–743. doi: 10.1097/00006534-199703000-00020. [DOI] [PubMed] [Google Scholar]
- Smith A. R., van Alphen W. A., van der Pompe W. B. Lymphatic drainage in patients after replantation of extremities. Plast Reconstr Surg. 1987 Feb;79(2):163–170. doi: 10.1097/00006534-198702000-00001. [DOI] [PubMed] [Google Scholar]
- Swartz M. A., Berk D. A., Jain R. K. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996 Jan;270(1 Pt 2):H324–H329. doi: 10.1152/ajpheart.1996.270.1.H324. [DOI] [PubMed] [Google Scholar]