Abstract
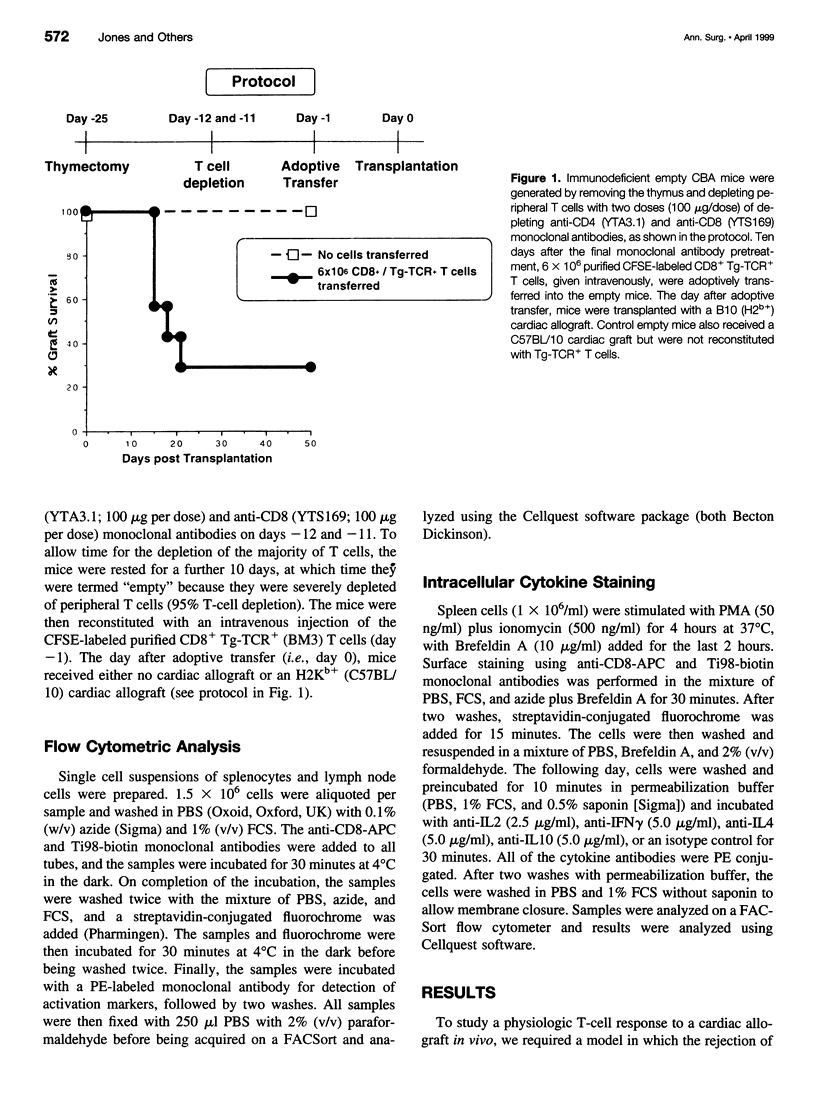
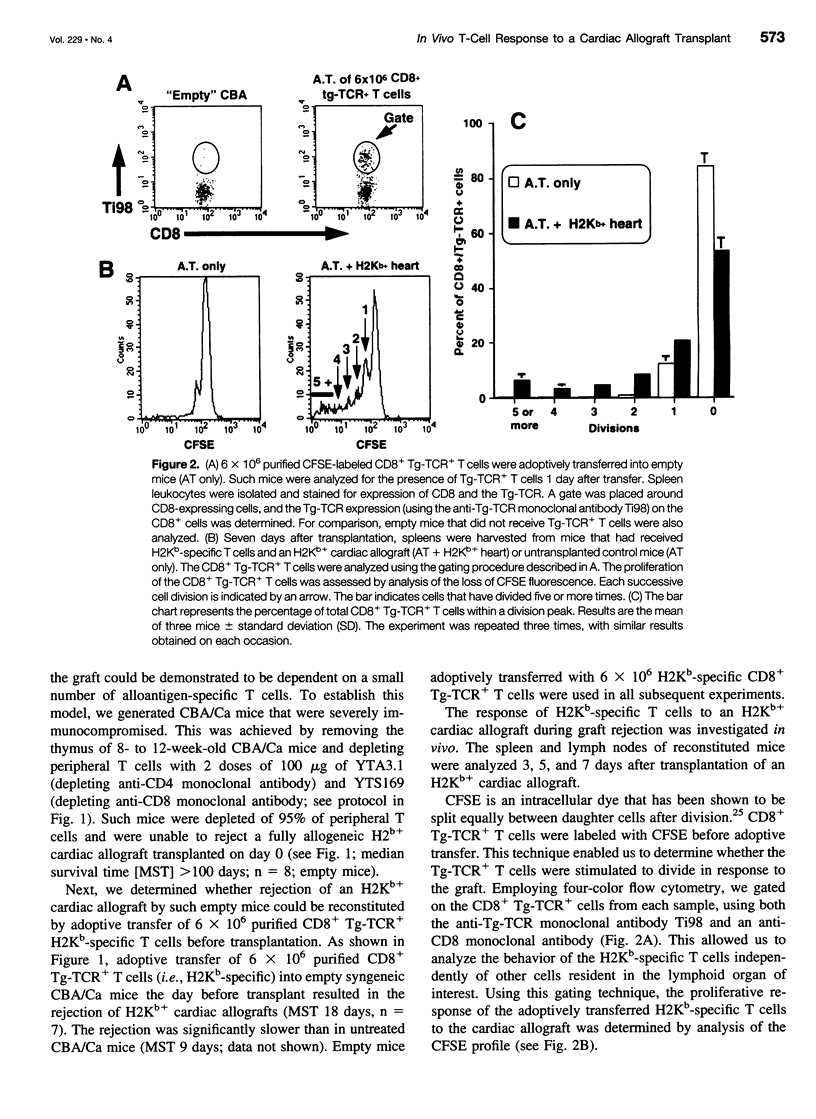
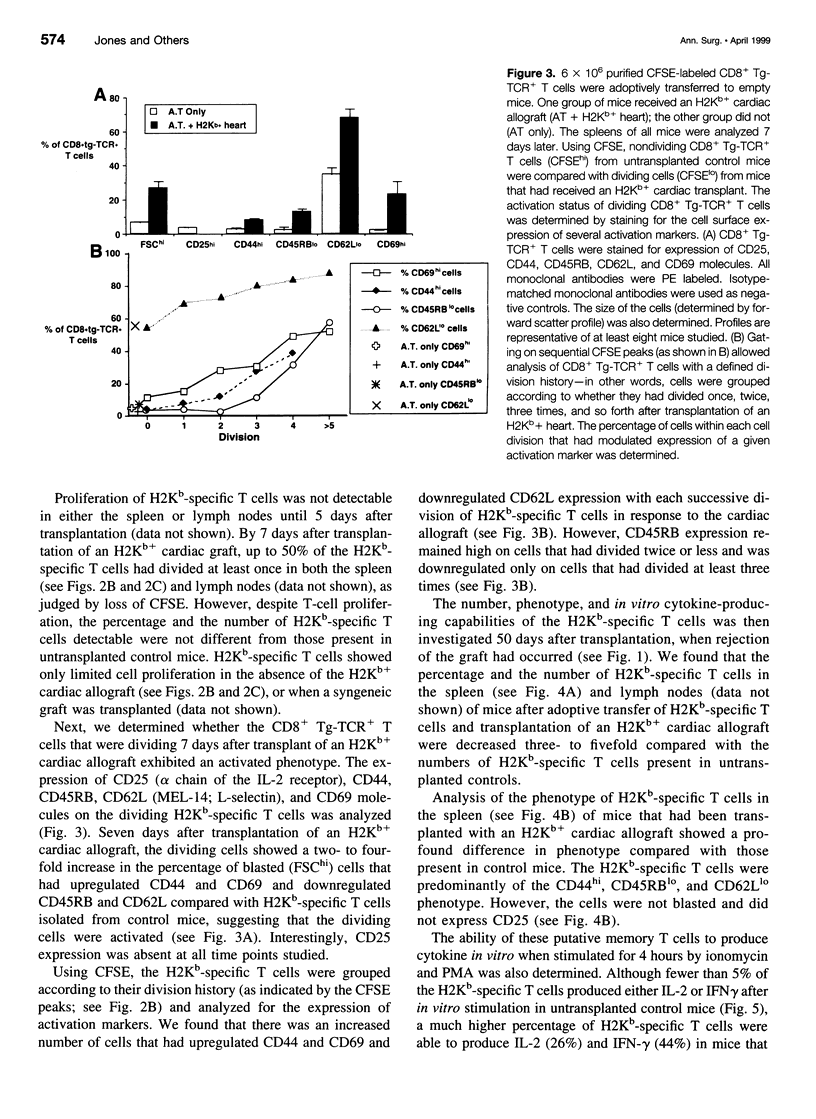
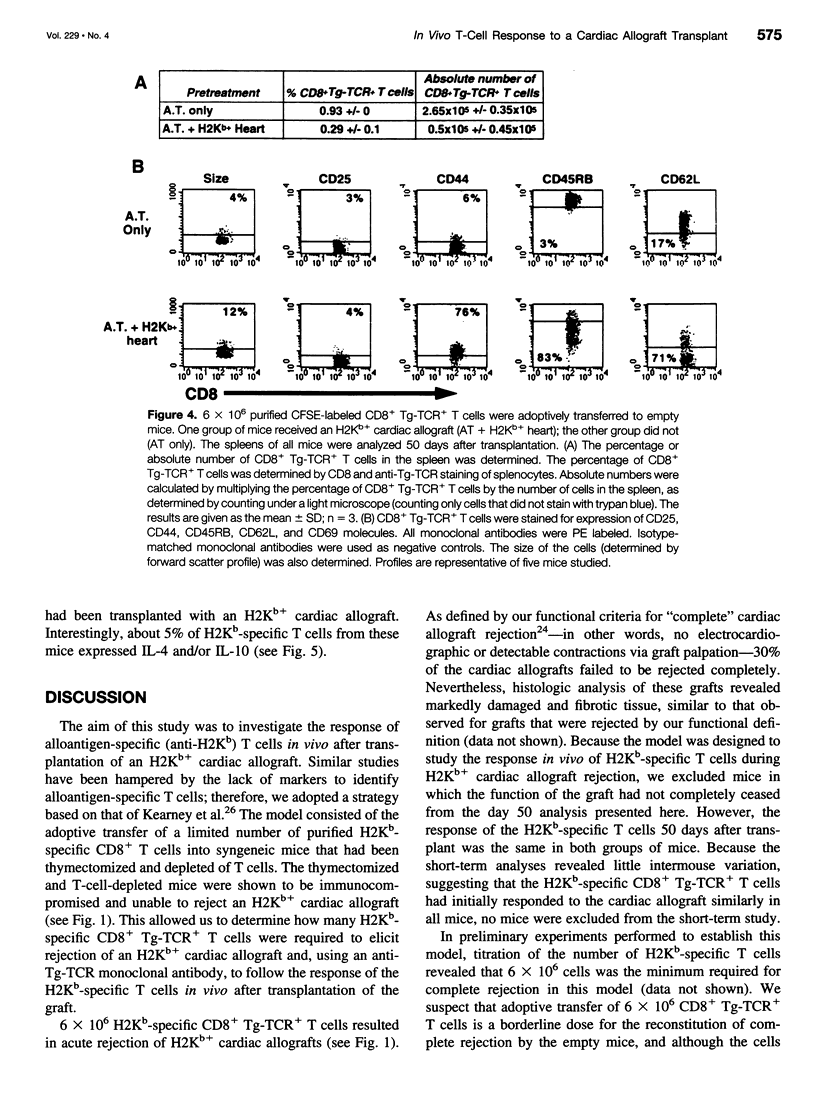
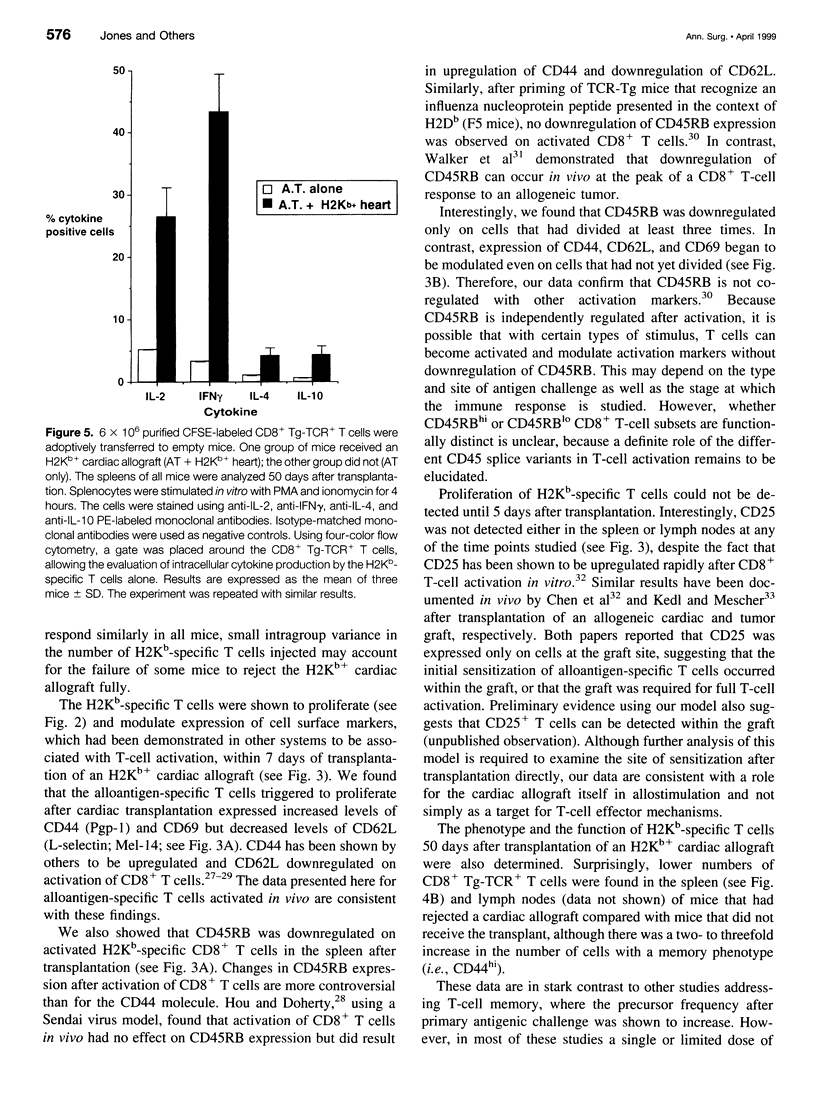
OBJECTIVE: To study the response of alloantigen (H2Kb)-specific T cells to a H2b+ cardiac allograft in vivo. SUMMARY BACKGROUND DATA: The response of T cells to alloantigen has been well characterized in vitro but has proved more difficult to assess in vivo. The aim of these experiments was to develop a model of T-cell-mediated rejection where the response of T cells after transplantation of a cardiac allograft could be followed in vivo. METHODS: Purified CD8+ T cells from H2Kb-specific TCR transgenic mice (BM3; H2k) were adoptively transferred into thymectomized, T-cell-depleted CBA/Ca (H2k) mice. These mice were then transplanted with a H2Kb+ cardiac allograft. Using four-color flow cytometry, the proliferative response, modulation of activation markers, and potential cytokine production of the H2Kb-specific T cells was assessed after transplantation. RESULTS: Consistent rejection of H2Kb+ cardiac allografts required the transfer of at least 6 x 10(6) CD8+ H2Kb-specific T cells. Short-term analyses revealed that the transgenic-TCR+/ CD8+ T cells proliferated and became activated after transplantation of an H2Kb+ cardiac allograft. Fifty days after transplantation, the transgenic-TCR+/CD8+ T cells remained readily detectable, bore a predominantly memory phenotype (CD44hi), and rapidly produced interleukin 2 and interferon-gamma on in vitro restimulation. CONCLUSIONS: These data show that the activation of alloantigen-specific T cells can be followed in vivo in short-term and long-term experiments, thereby providing a unique opportunity to study the mechanisms by which T cells respond to allografts in vivo.
Full text
PDF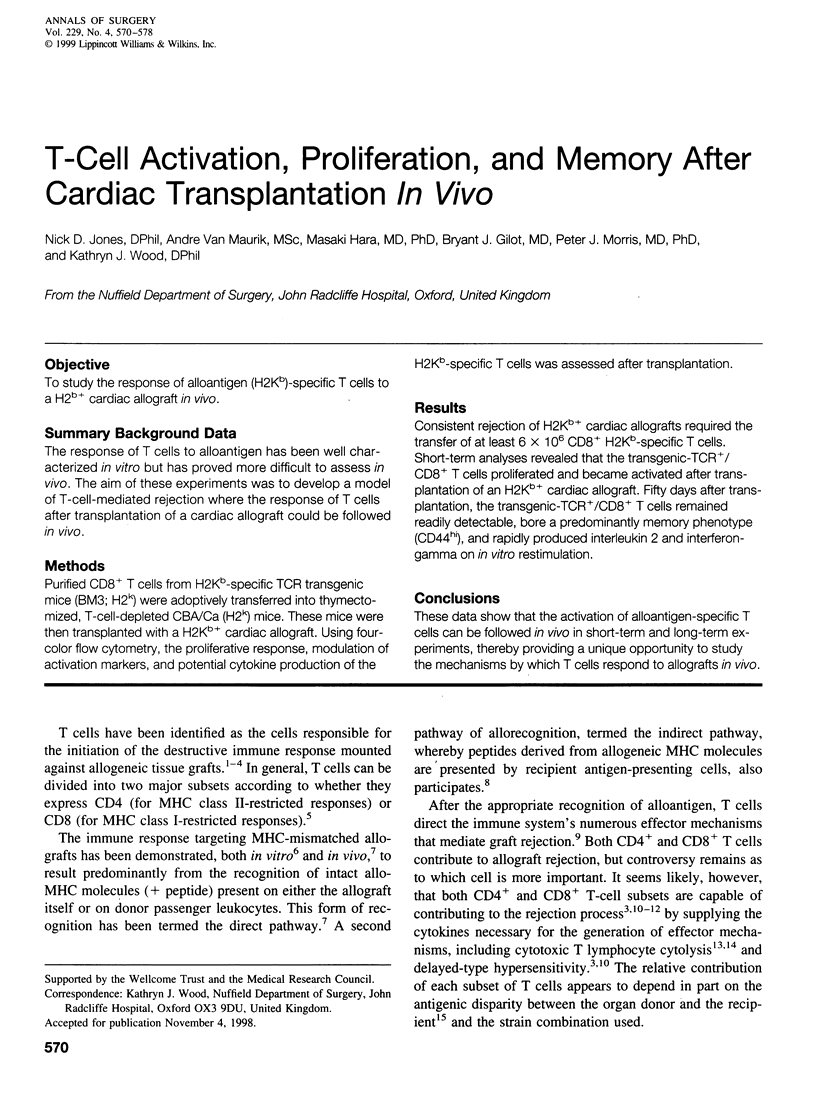



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auphan N., Curnow J., Guimezanes A., Langlet C., Malissen B., Mellor A., Schmitt-Verhulst A. M. The degree of CD8 dependence of cytolytic T cell precursors is determined by the nature of the T cell receptor (TCR) and influences negative selection in TCR-transgenic mice. Eur J Immunol. 1994 Jul;24(7):1572–1577. doi: 10.1002/eji.1830240718. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Bishop D. K., Chan S., Li W., Ensley R. D., Xu S., Eichwald E. J. CD4-positive helper T lymphocytes mediate mouse cardiac allograft rejection independent of donor alloantigen specific cytotoxic T lymphocytes. Transplantation. 1993 Oct;56(4):892–897. doi: 10.1097/00007890-199310000-00023. [DOI] [PubMed] [Google Scholar]
- Braun M. Y., McCormack A., Webb G., Batchelor J. R. Mediation of acute but not chronic rejection of MHC-incompatible rat kidney grafts by alloreactive CD4 T cells activated by the direct pathway of sensitization. Transplantation. 1993 Jan;55(1):177–182. doi: 10.1097/00007890-199301000-00033. [DOI] [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., Horvath C., Bron C., Pedrazzini T., Howe R. C., MacDonald H. R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987 May 15;138(10):3120–3129. [PubMed] [Google Scholar]
- Buferne M., Luton F., Letourneur F., Hoeveler A., Couez D., Barad M., Malissen B., Schmitt-Verhulst A. M., Boyer C. Role of CD3 delta in surface expression of the TCR/CD3 complex and in activation for killing analyzed with a CD3 delta-negative cytotoxic T lymphocyte variant. J Immunol. 1992 Feb 1;148(3):657–664. [PubMed] [Google Scholar]
- Chen H., Luo H., Xu D., Loh D. Y., Daloze P. M., Veillette A., Qi S., Wu J. Impaired signaling in alloantigen-specific CD8+ T cells tolerized in vivo: employing a model of Ld-specific TCR transgenic mice transplanted with allogenic hearts under the cover of a short-term rapamycin treatment. J Immunol. 1996 Nov 15;157(10):4297–4308. [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W. Role of thymus-derived and thymus-independent cells in murine skin allograft rejection. Transplantation. 1982 Mar;33(3):221–223. doi: 10.1097/00007890-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Hall B. M., Dorsch S., Roser B. The cellular basis of allograft rejection in vivo. I. The cellular requirements for first-set rejection of heart grafts. J Exp Med. 1978 Oct 1;148(4):878–889. doi: 10.1084/jem.148.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Doherty P. C. Partitioning of responder CD8+ T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J Immunol. 1993 Jun 15;150(12):5494–5500. [PubMed] [Google Scholar]
- Hua C., Boyer C., Guimezanes A., Albert F., Schmitt-Verhulst A. M. Analysis of T cell activation requirements with the use of alloantigens or an anti-clonotypic monoclonal antibody. J Immunol. 1986 Mar 15;136(6):1927–1936. [PubMed] [Google Scholar]
- Ilano A. L., McConnell M. V., Gurley K. E., Spinelli A., Pearce N. W., Hall B. M. Cellular basis of allograft rejection in vivo. V. Examination of the mechanisms responsible for the differing efficacy of monoclonal antibody to CD4+ T cell subsets in low- and high-responder rat strains. J Immunol. 1989 Nov 1;143(9):2828–2836. [PubMed] [Google Scholar]
- Kearney E. R., Pape K. A., Loh D. Y., Jenkins M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994 Jul;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Kedl R. M., Mescher M. F. Migration and activation of antigen-specific CD8+ T cells upon in vivo stimulation with allogeneic tumor. J Immunol. 1997 Jul 15;159(2):650–663. [PubMed] [Google Scholar]
- Lau L. L., Jamieson B. D., Somasundaram T., Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994 Jun 23;369(6482):648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., McKenzie I. F. Which T cells cause graft rejection? Transplantation. 1982 Mar;33(3):217–221. doi: 10.1097/00007890-198203000-00001. [DOI] [PubMed] [Google Scholar]
- Lyons A. B., Parish C. R. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994 May 2;171(1):131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Mobley J. L., Rigby S. M., Dailey M. O. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994 Dec 15;153(12):5443–5452. [PubMed] [Google Scholar]
- Pearson T. C., Hamano K., Morris P. J., Wood K. J. Anti-CD4 monoclonal antibody-induced allograft survival is associated with a defect in interleukin-2-dependent T-cell activation. Transplant Proc. 1993 Feb;25(1 Pt 1):786–787. [PubMed] [Google Scholar]
- Pihlgren M., Lightstone L., Mamalaki C., Rimon G., Kioussis D., Marvel J. Expression in vivo of CD45RA, CD45RB and CD44 on T cell receptor-transgenic CD8+ T cells following immunization. Eur J Immunol. 1995 Jun;25(6):1755–1759. doi: 10.1002/eji.1830250640. [DOI] [PubMed] [Google Scholar]
- Rocha B., Grandien A., Freitas A. A. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995 Mar 1;181(3):993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Singer A. Analysis of T-cell subsets in rejection of Kb mutant skin allografts differing at class I MHC. 1986 Aug 28-Sep 3Nature. 322(6082):829–831. doi: 10.1038/322829a0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Wagner H., Harris A. W. In vivo activity of in vitro immunized lymphocytes. I. Tumor allograft rejection mediated by in vitro activated mouse thymocytes. J Immunol. 1972 May;108(5):1353–1361. [PubMed] [Google Scholar]
- Rouse B. T., Wagner H. The in vivo activity of in vitro immunized mouse thymocytes. II. Rejection of skin allografts and graft-vs-host activity. J Immunol. 1972 Dec;109(6):1282–1289. [PubMed] [Google Scholar]
- Shoskes D. A., Wood K. J. Indirect presentation of MHC antigens in transplantation. Immunol Today. 1994 Jan;15(1):32–38. doi: 10.1016/0167-5699(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Sponaas A. M., Tomlinson P. D., Antoniou J., Auphan N., Langlet C., Malissen B., Schmitt-Verhulst A. M., Mellor A. L. Induction of tolerance to self MHC class I molecules expressed under the control of milk protein or beta-globin gene promoters. Int Immunol. 1994 Feb;6(2):277–287. doi: 10.1093/intimm/6.2.277. [DOI] [PubMed] [Google Scholar]
- Superina R. A., Peugh W. N., Wood K. J., Morris P. J. Assessment of primarily vascularized cardiac allografts in mice. Transplantation. 1986 Aug;42(2):226–227. [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Ohteki T., Lopez J. A., MacDonald H. R., Maryanski J. L. Distinct phenotypes of antigen-selected CD8 T cells emerge at different stages of an in vivo immune response. J Immunol. 1995 Oct 1;155(7):3443–3452. [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]


