Abstract
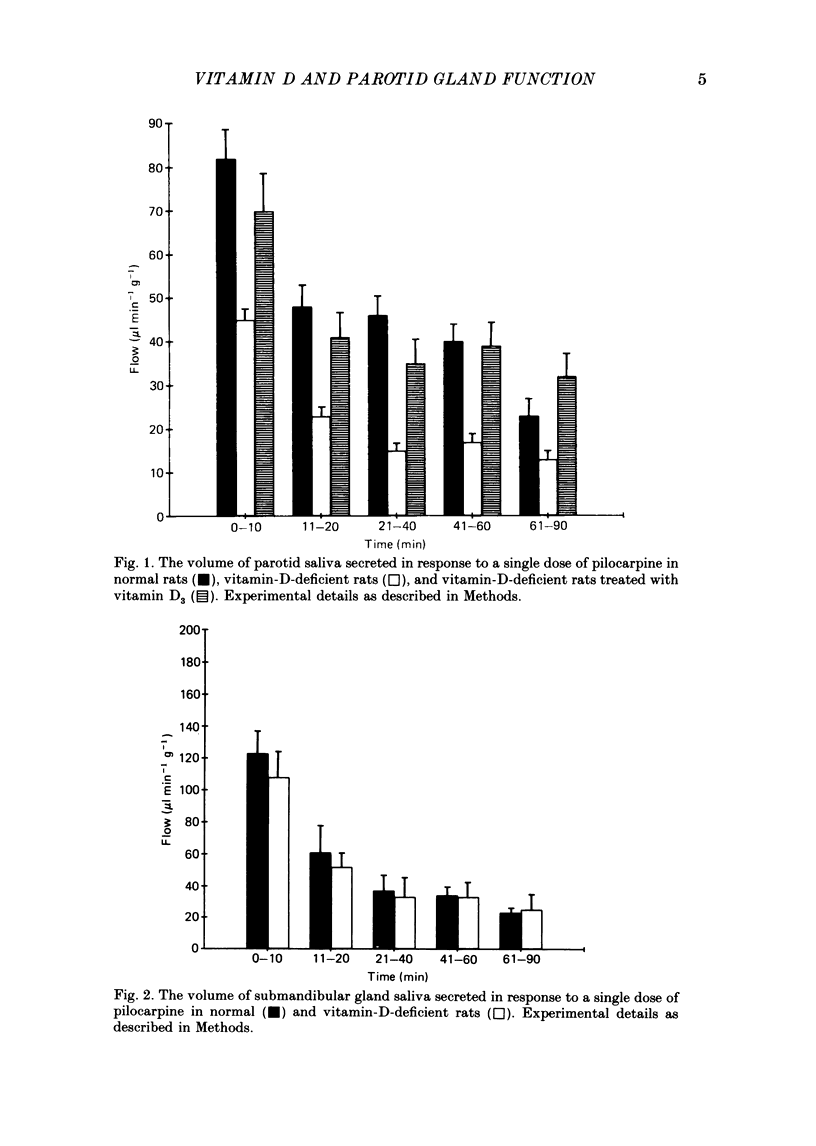
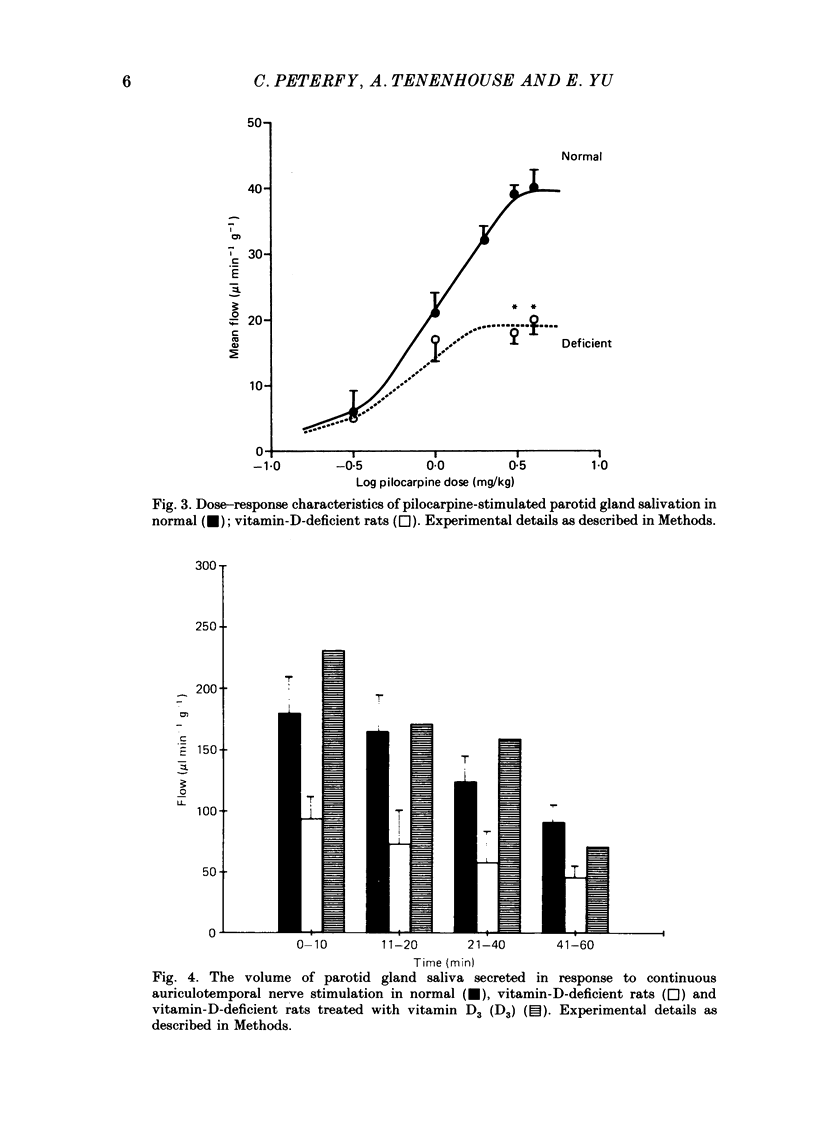
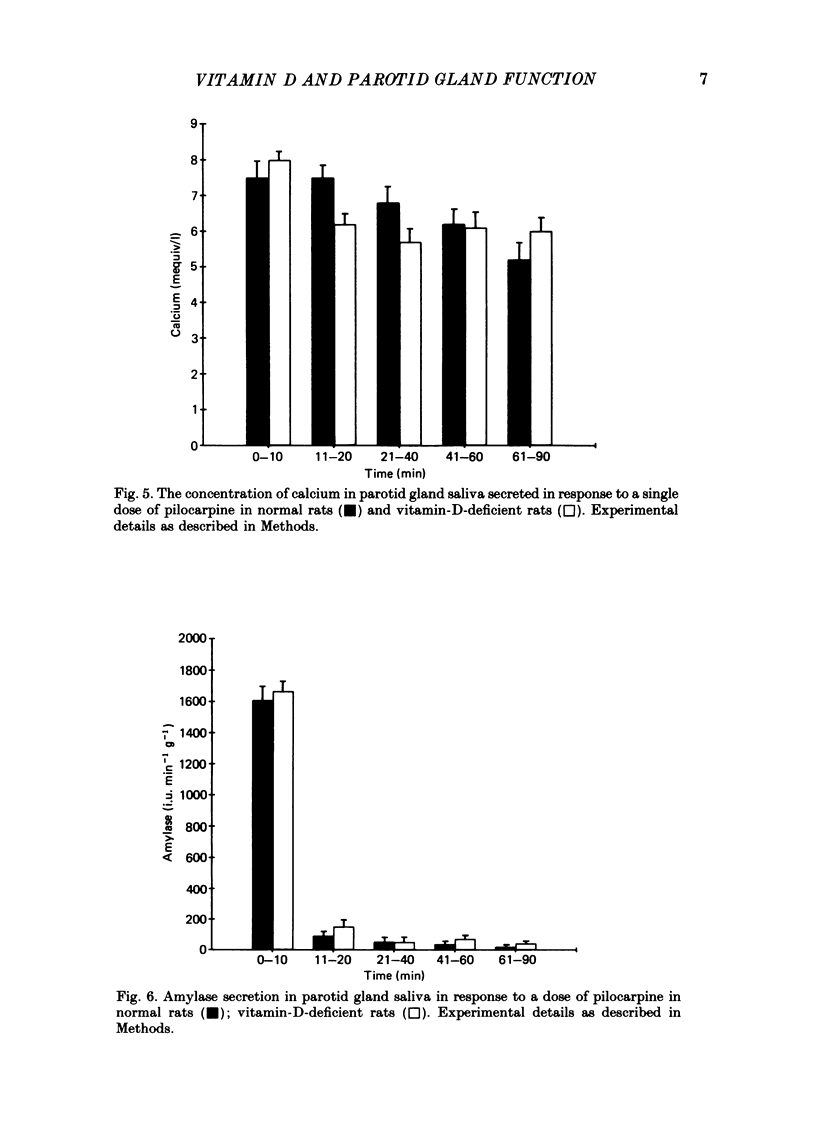
1. We previously reported that parotid gland secretion is decreased in rats deprived of vitamin D (Glijer, Peterfy & Tenenhouse, 1985). In the present study we examine whether this effect is a direct result of the absence of vitamin D or due to the secondary systemic effects of vitamin D deficiency. 2. Offspring of rats maintained on a calcium-supplemented (1.2%), vitamin-D-deficient diet were weaned onto the same diet and examined after 8 weeks. Using this method it was possible to maintain serum calcium and parathyroid hormone concentrations within normal limits. Serum 25-hydroxyvitamin D (25(OH)D3) was not detectable, but 1,25-dihydroxyvitamin D (1,25(OH)2D3) concentrations were normal. 3. Pilocarpine-stimulated flow of parotid saliva was reduced 57% in vitamin-D-deprived animals, but amylase secretion was unchanged. Treatment with vitamin D3 returned flow rates to normal. 4. The concentration of calcium in parotid saliva was normal in vitamin-D-deprived rats, although total parotid calcium output was reduced 57%. 5. Pilocarpine-stimulated salivary flow from submandibular gland, a tissue which does not possess 1,25(OH)2D3 receptors, was normal in vitamin-D-deprived rats. 6. Heart rate and arterial blood pressure changes in response to I.V. pilocarpine administration were identical in normal and vitamin-D-deficient rats. 7. Auriculotemporal nerve-stimulated flow of parotid saliva was also reduced by 50% and administration of vitamin D3 to these rats corrected this abnormality. 8. It is concluded that fluid and electrolyte secretion from parotid gland is directly dependent on vitamin D; abnormal parotid gland function seen in vitamin-D-deficient rats is not due to secondary hypocalcaemia or hyperparathyroidism, nor can it be explained by haemodynamic changes evoked during systemic administration of pilocarpine. We further conclude that the metabolite of vitamin D responsible for this effect is not 1,25(OH)2D3.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P. O., Bloom S. R., Edwards A. V., Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982 Jan;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H., Hayakawa M., Takiguchi H. Some characteristics of cytosol binding protein for 1 alpha,25-dihydroxycholecalciferol, 24R,25-dihydroxycholecalciferol and 25-hydroxycholecalciferol in rat parotid gland. Int J Biochem. 1982;14(8):705–712. doi: 10.1016/0020-711x(82)90006-4. [DOI] [PubMed] [Google Scholar]
- Brommage R., DeLuca H. F. Evidence that 1,25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocr Rev. 1985 Fall;6(4):491–511. doi: 10.1210/edrv-6-4-491. [DOI] [PubMed] [Google Scholar]
- Butcher F. R. Regulation of calcium efflux from isolated rat parotid cells. Biochim Biophys Acta. 1980 Jun 19;630(2):254–260. doi: 10.1016/0304-4165(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Ceska M., Brown B., Birath K. Ranges of alpha-amylase activities in human serum and urine and correlations with some other alpha-amylase methods. Clin Chim Acta. 1969 Dec;26(3):445–453. doi: 10.1016/0009-8981(69)90072-2. [DOI] [PubMed] [Google Scholar]
- Ekström J., Brodin E., Ekman R., Håkanson R., Månsson B., Tobin G. Depletion of neuropeptides in rat parotid glands and declining atropine-resistant salivary secretion upon continuous parasympathetic nerve stimulation. Regul Pept. 1985 Aug;11(4):353–359. doi: 10.1016/0167-0115(85)90207-1. [DOI] [PubMed] [Google Scholar]
- Gamblin G. T., Liberman U. A., Eil C., Downs R. W., Jr, DeGrange D. A., Marx S. J. Vitamin D-dependent rickets type II. Defective induction of 25-hydroxyvitamin D3-24-hydroxylase by 1,25-dihydroxyvitamin D3 in cultured skin fibroblasts. J Clin Invest. 1985 Mar;75(3):954–960. doi: 10.1172/JCI111796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glijer B., Peterfy C., Tenenhouse A. The effect of vitamin D deficiency on secretion of saliva by rat parotid gland in vivo. J Physiol. 1985 Jun;363:323–334. doi: 10.1113/jphysiol.1985.sp015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D., Noff D., Edelstein S. The parotid gland: a new target organ for vitamin D action. Biochim Biophys Acta. 1978 Mar 1;539(2):249–252. doi: 10.1016/0304-4165(78)90011-9. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Aoki H., Terao N., Abiko Y., Takiguchi H. Vitamin D-mediated decrease of Ca2+-pump activity in the rat parotid gland. Int J Biochem. 1983;15(9):1175–1178. doi: 10.1016/0020-711x(83)90234-3. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Turner R. T., Sherrard D. J., Baylink D. J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981 Aug 10;256(15):7738–7740. [PubMed] [Google Scholar]
- Kumar R., Schnoes H. K., DeLuca H. F. Rat intestinal 25-hydroxyvitamin D3- and 1alpha,25-dihydroxyvitamin D3-24-hydroxylase. J Biol Chem. 1978 Jun 10;253(11):3804–3809. [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J., Hökfelt T., Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Peterfy C., Tenenhouse A. Vitamin D receptors in isolated rat parotid gland acinar cells. Biochim Biophys Acta. 1982 Oct 11;721(2):158–163. doi: 10.1016/0167-4889(82)90063-5. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Norman A. W. Regulation of 25-hydroxyvitamin D3 metabolism in a human promyelocytic leukemia cell line (HL-60): 1,25-dihydroxyvitamin D3 stimulates the synthesis of 24,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1986 Nov 15;251(1):222–231. doi: 10.1016/0003-9861(86)90069-x. [DOI] [PubMed] [Google Scholar]
- Schachter M. Kallikreins and kinins. Physiol Rev. 1969 Jul;49(3):509–547. doi: 10.1152/physrev.1969.49.3.509. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Growth pattern of postnatally developing rat parotid gland. Proc Soc Exp Biol Med. 1969 Feb;130(2):603–607. doi: 10.3181/00379727-130-33617. [DOI] [PubMed] [Google Scholar]
- Schneyer C. A., Hall H. D. Time course and autonomic regulation of development of secretory function of rat parotid. Am J Physiol. 1968 Apr;214(4):808–813. doi: 10.1152/ajplegacy.1968.214.4.808. [DOI] [PubMed] [Google Scholar]
- Taft J. L., Shaw M., Danks J. A., Prince R. L., Larkins R. G. A role for calmodulin in renal production of 1,25-dihydroxyvitamin D3. Endocrinology. 1986 Sep;119(3):1131–1136. doi: 10.1210/endo-119-3-1131. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974 Mar;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- Tenenhouse A., Afari G. Protein secretion from parotid glands of vitamin D deficient and glucocorticoid-treated rats. Biochim Biophys Acta. 1978 Feb 1;538(3):631–634. doi: 10.1016/0304-4165(78)90424-5. [DOI] [PubMed] [Google Scholar]
- Warner M., Tenenhouse A. Regulation of renal vitamin D hydroxylase activity in vitamin D deficient rats. Can J Physiol Pharmacol. 1985 Aug;63(8):978–982. doi: 10.1139/y85-161. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Fullmer C. S. Calcium transport proteins, calcium absorption, and vitamin D. Annu Rev Physiol. 1983;45:375–390. doi: 10.1146/annurev.ph.45.030183.002111. [DOI] [PubMed] [Google Scholar]
- Winick M., Noble A. Quantitative changes in DNA, RNA, and protein during prenatal and postnatal growth in the rat. Dev Biol. 1965 Dec;12(3):451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]


