Abstract
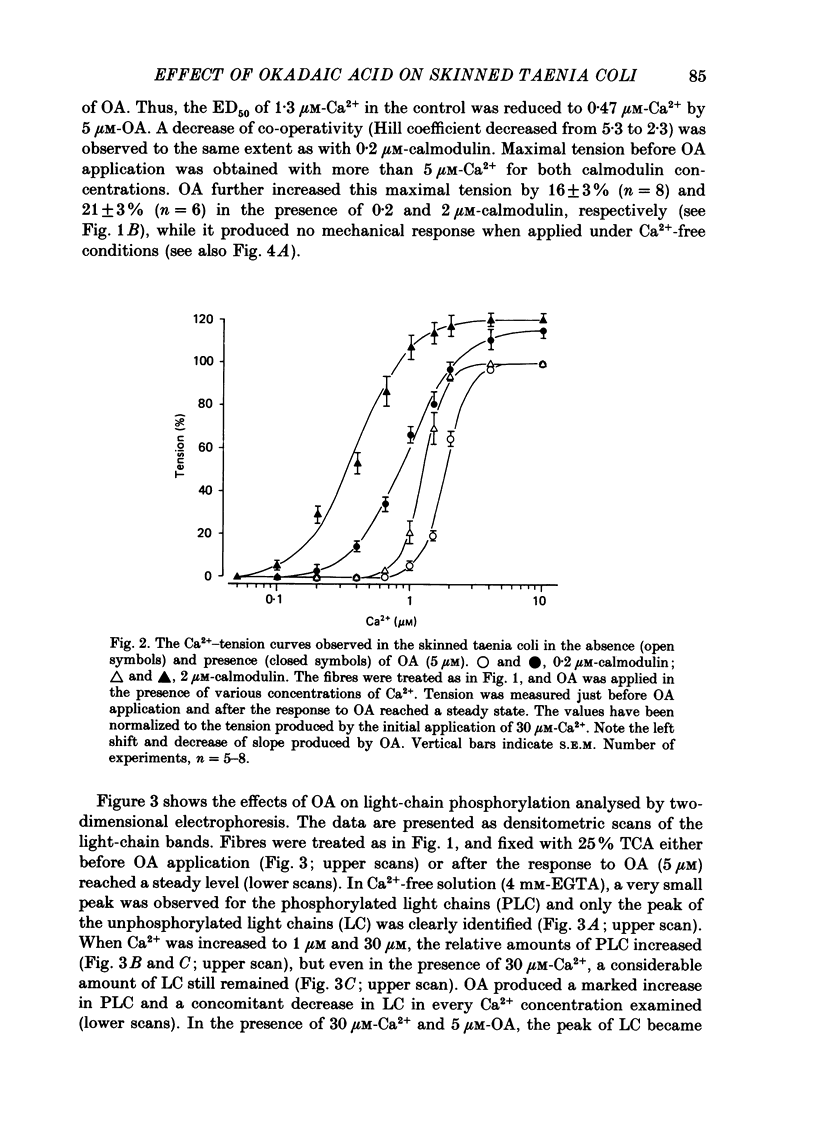
1. In guinea-pig taenia coli skinned with Triton X-100, the marine sponge toxin okadaic acid (OA; 0.1-10 microM) produced a dose-dependent enhancement of isometric tension in the presence of low concentrations (0.1-1 microM) of Ca2+. 2. The Ca2+-tension relation of the skinned taenia showed a high co-operativity (Hill coefficient, h = 5) in the presence of 0.2 microM-calmodulin. The concentration of Ca2+ required to obtain half-maximal tension (ED50) was 1.8 microM. OA (5 microM) reduced the co-operativity (h = 2.3) and increased the Ca2+ sensitivity (ED50 = 0.92 microM-Ca2+). OA further increased the tension produced with 30 microM-Ca2+, while it failed to produce any mechanical effect in Ca2+-free solution. When the calmodulin concentration was increased the Ca2+ sensitivity increased as well, but the co-operativity was not affected both in the absence and in the presence of OA. 3. The level of myosin phosphorylation was analysed by two-dimensional gel electrophoresis. OA produced an increase in phosphorylated light chains and a concomitant decrease in unphosphorylated light chains. The effect was completely reversed when OA was washed out. 4. In solutions containing more than 1 microM-Ca2+, a third protein band appeared on the gels next to the bands of light chains. OA markedly increased the third band which disappeared when OA and Ca2+ were simultaneously removed. 5. OA reversibly slowed down both relaxation and dephosphorylation induced by Ca2+ removal following activation with 30 microM-Ca2+. Complete relaxation did not occur in the presence of more than 1 microM-OA. The concentration of OA required to produce a 50% reduction (ID50) of the relaxation rate was 78 nM. 6. The phosphatase activity in the taenia extract was inhibited by OA (1-10 microM) in a dose-dependent manner. The inhibition was well described as a mixed noncompetitive inhibition, and the dose-inhibition relation was shifted to the right when the concentration of substrate (phosphorylated light chains) was increased. The lower and upper limits of the change of ID50 produced by changing the substrate concentration were estimated to be 10 and 165 nM-OA, respectively. 7. These results strongly suggest that the tension enhancement and the slow-down of relaxation are both causally related to inhibition of myosin phosphatase activity by OA.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Cole H. A., Patchell V. B., Perry S. V. Phosphorylation of chicken gizzard myosin and the Ca2+-sensitivity of the actin-activated Mg2+-ATPase. FEBS Lett. 1983 Jul 11;158(1):17–20. doi: 10.1016/0014-5793(83)80667-x. [DOI] [PubMed] [Google Scholar]
- Di Salvo J., Gifford D., Kokkinakis A. A new heat-stable regulatory factor is associated with aortic polycation-modulated (PCM)-phosphatase. Proc Soc Exp Biol Med. 1985 Dec;180(3):488–496. doi: 10.3181/00379727-180-42207. [DOI] [PubMed] [Google Scholar]
- DiSalvo J., Gifford D., Jiang M. J. Properties and function of phosphatases from vascular smooth muscle. Fed Proc. 1983 Jan;42(1):67–71. [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagelmann M., Rüegg J. C., Di Salvo J. Phosphorylation of the myosin light chains and satellite proteins in detergent-skinned arterial smooth muscle. Biochem Biophys Res Commun. 1984 May 16;120(3):933–938. doi: 10.1016/s0006-291x(84)80196-5. [DOI] [PubMed] [Google Scholar]
- Güth K., Junge J. Low Ca2+ impedes cross-bridge detachment in chemically skinned Taenia coli. Nature. 1982 Dec 23;300(5894):775–776. doi: 10.1038/300775a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mrwa U. Regulation of smooth muscle actomyosin. Blood Vessels. 1982;19(1):1–18. doi: 10.1159/000158369. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama I., Kondo N., Shibata S. Electromechanical effects of okadaic acid isolated from black sponge in guinea-pig ventricular muscles. J Physiol. 1986 Sep;378:359–373. doi: 10.1113/jphysiol.1986.sp016224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ozaki H., Kohama K., Nonomura Y., Shibata S., Karaki H. Direct activation by okadaic acid of the contractile elements in the smooth muscle of guinea-pig taenia coli. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):356–358. doi: 10.1007/BF00172811. [DOI] [PubMed] [Google Scholar]
- Pato M. D., Adelstein R. S., Crouch D., Safer B., Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 4. Classification of two homogeneous myosin light chain phosphatases from smooth muscle as protein phosphatase-2A1 and 2C, and a homogeneous protein phosphatase from reticulocytes active on protein synthesis initiation factor eIF-2 as protein phosphatase-2A2. Eur J Biochem. 1983 May 2;132(2):283–287. doi: 10.1111/j.1432-1033.1983.tb07360.x. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Hofmann F., DiSalvo J., Rüegg J. C. cGMP and cAMP inhibit tension development in skinned coronary arteries. Pflugers Arch. 1984 Jul;401(3):277–280. doi: 10.1007/BF00582596. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Merkel L., Rüegg J. C., Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers Arch. 1986 Jul;407(1):87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Rüegg J. C., Zimmer M., Hofmann F. Relaxation of skinned coronary arteries depends on the relative concentrations of Ca2+, calmodulin and active cAMP-dependent protein kinase. Pflugers Arch. 1985 Sep;405(1):70–76. doi: 10.1007/BF00591100. [DOI] [PubMed] [Google Scholar]
- Porter W. R., Trager W. F. Improved non-parametric statistical methods for the estimation of Michaelis-Menten kinetic parameters by the direct linear plot. Biochem J. 1977 Feb 1;161(2):293–302. doi: 10.1042/bj1610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Sparrow M., Rüegg J. C. Inorganic phosphate promotes relaxation of chemically skinned smooth muscle of guinea-pig Taenia coli. Experientia. 1981;37(9):980–982. doi: 10.1007/BF01971791. [DOI] [PubMed] [Google Scholar]
- Shibata S., Ishida Y., Kitano H., Ohizumi Y., Habon J., Tsukitani Y., Kikuchi H. Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of Ca deficiency. J Pharmacol Exp Ther. 1982 Oct;223(1):135–143. [PubMed] [Google Scholar]
- Takai A., Bialojan C., Troschka M., Rüegg J. C. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987 Jun 8;217(1):81–84. doi: 10.1016/0014-5793(87)81247-4. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]


