Abstract
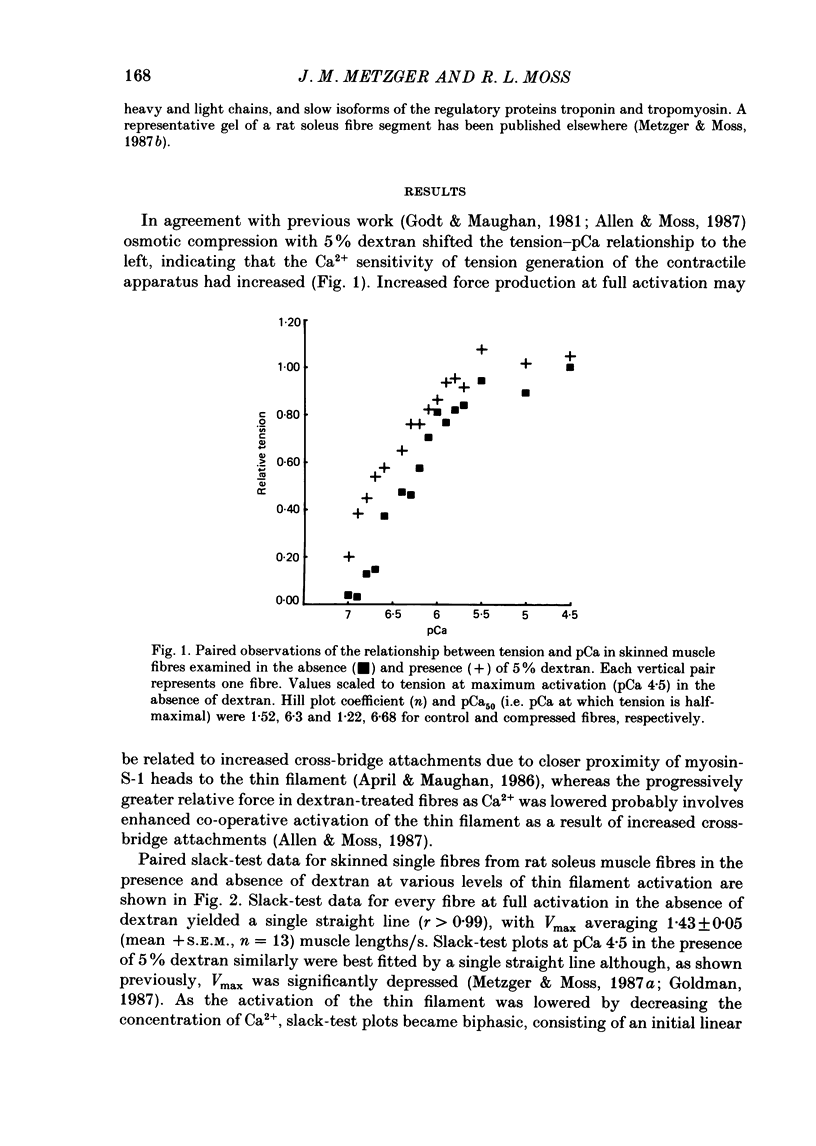
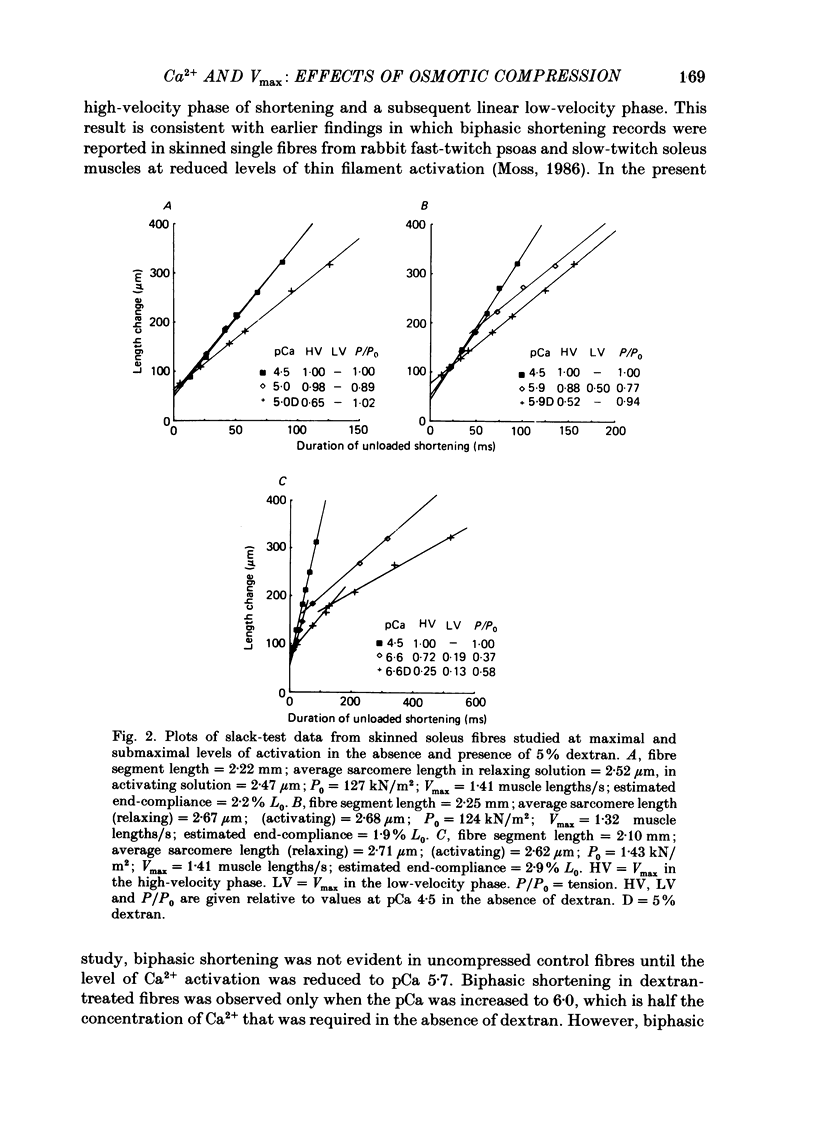
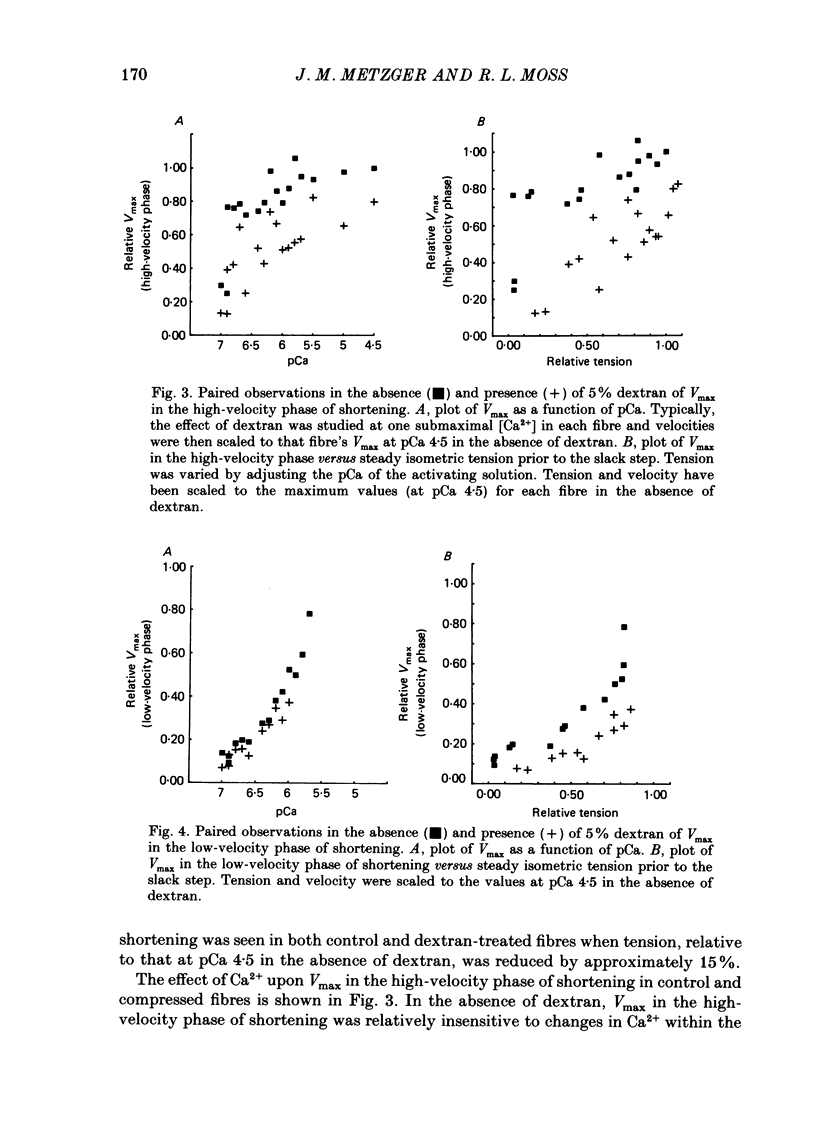
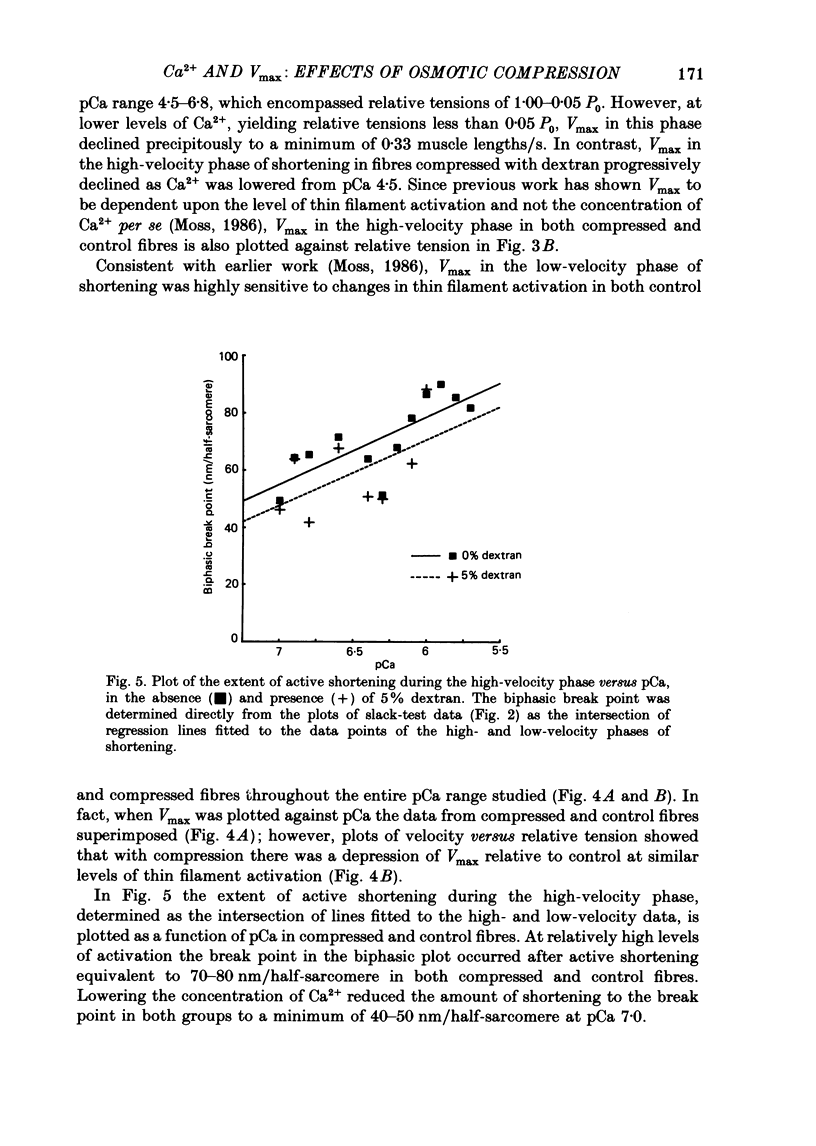
1. Maximum shortening velocity (Vmax) was examined in skinned single fibres from rat slow-twitch soleus muscles at various degrees of Ca2+ activation of the thin filament, in the presence and absence of osmotic compression induced by 5% dextran. 2. At maximal levels of Ca2+ activation, Vmax remained constant as the extent of shortening was varied, with values averaging 1.43 +/- 0.05 muscle lengths/s (mean + S.E.M., n = 13). When thin filament activation was reduced by lowering the concentration of Ca2+, unloaded shortening consisted of an initial high-velocity phase for extents of shortening in the range 20-80 nm/half-sarcomere, and a subsequent low-velocity phase for greater extents of shortening. 3. In the absence of dextran, Vmax in the high-velocity phase of shortening was relatively invariant over a wide range of activation; however, at very low levels of activation, yielding tensions less than 5% of the peak value. Vmax declined precipitously. In contrast, fibres compressed radially with dextran to diameters comparable to those of intact fibres demonstrated a marked decrease in Vmax in the high-velocity phase when thin filament activation was varied over a wide range. These findings are consistent with the idea that cross-bridges in the expanded filament lattice of skinned fibres do not bear as great an axial compressive force as in intact fibres (Goldman & Simmons, 1986; Goldman, 1987) but instead buckle as the fibre shortens. 4. The value of Vmax in the low-velocity phase of shortening decreased as thin filament activation was reduced in both control and osmotically compressed fibres. The low-velocity phase, which occurred only at reduced levels of thin filament activation, may be a manifestation of a population of slowly dissociating crossbridges which with shortening become negatively strained and oppose contraction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. D., Moss R. L. Factors influencing the ascending limb of the sarcomere length-tension relationship in rabbit skinned muscle fibres. J Physiol. 1987 Sep;390:119–136. doi: 10.1113/jphysiol.1987.sp016689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April E. W., Maughan D. W. Active force as a function of filament spacing in crayfish skinned muscle fibers. Pflugers Arch. 1986 Oct;407(4):456–460. doi: 10.1007/BF00652634. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Rutchik J. S., Schachat F. H. Co-operative interactions between troponin-tropomyosin units extend the length of the thin filament in skeletal muscle. J Mol Biol. 1987 Jun 20;195(4):885–896. doi: 10.1016/0022-2836(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Schachat F. H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984 Dec 5;180(2):379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981 Oct;391(4):334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E. Measurement of sarcomere shortening in skinned fibers from frog muscle by white light diffraction. Biophys J. 1987 Jul;52(1):57–68. doi: 10.1016/S0006-3495(87)83188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. The stiffness of frog skinned muscle fibres at altered lateral filament spacing. J Physiol. 1986 Sep;378:175–194. doi: 10.1113/jphysiol.1986.sp016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Contraction kinetics of intact and skinned frog muscle fibers and degree of activation. Effects of intracellular Ca2+ on unloaded shortening. J Gen Physiol. 1985 Oct;86(4):479–500. doi: 10.1085/jgp.86.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The influence of free calcium on the maximum speed of shortening in skinned frog muscle fibres. J Physiol. 1986 Nov;380:257–273. doi: 10.1113/jphysiol.1986.sp016284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J. 1987 Jul;52(1):127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Allen J. D., Greaser M. L. Effects of partial extraction of troponin complex upon the tension-pCa relation in rabbit skeletal muscle. Further evidence that tension development involves cooperative effects within the thin filament. J Gen Physiol. 1986 May;87(5):761–774. doi: 10.1085/jgp.87.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Effects on shortening velocity of rabbit skeletal muscle due to variations in the level of thin-filament activation. J Physiol. 1986 Aug;377:487–505. doi: 10.1113/jphysiol.1986.sp016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Effects of EDTA treatment upon the protein subunit composition and mechanical properties of mammalian single skeletal muscle fibers. J Cell Biol. 1983 Apr;96(4):970–978. doi: 10.1083/jcb.96.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Physiological effects accompanying the removal of myosin LC2 from skinned skeletal muscle fibers. J Biol Chem. 1982 Aug 10;257(15):8588–8591. [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. The effect of calcium on the maximum velocity of shortening in skinned skeletal muscle fibres of the rabbit. J Muscle Res Cell Motil. 1982 Sep;3(3):295–311. doi: 10.1007/BF00713039. [DOI] [PubMed] [Google Scholar]
- Podolin R. A., Ford L. E. Influence of partial activation on force-velocity properties of frog skinned muscle fibers in millimolar magnesium ion. J Gen Physiol. 1986 Apr;87(4):607–631. doi: 10.1085/jgp.87.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Gordon A. M., Martyn D. A. Hysteresis in the force-calcium relation in muscle. Science. 1983 Mar 4;219(4588):1075–1077. doi: 10.1126/science.6823567. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T., Arata T., Oosawa F. Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature. 1985 Jul 25;316(6026):366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]


