Abstract
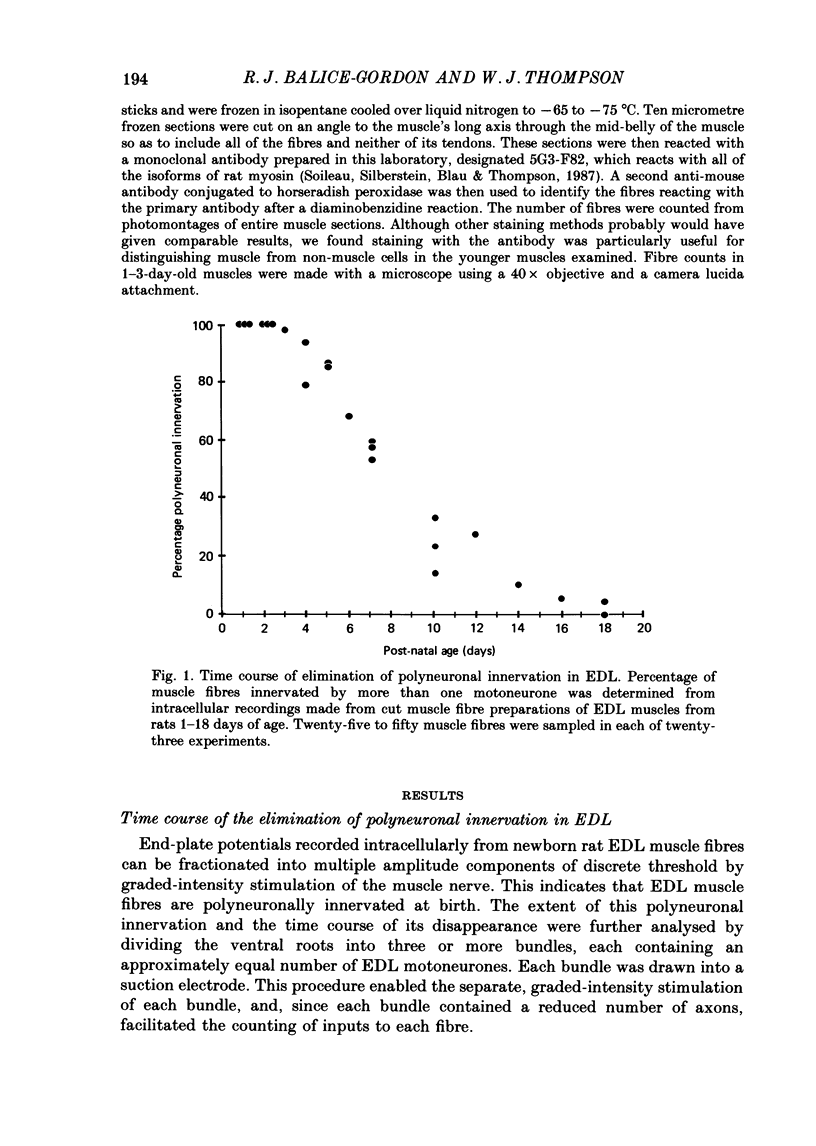
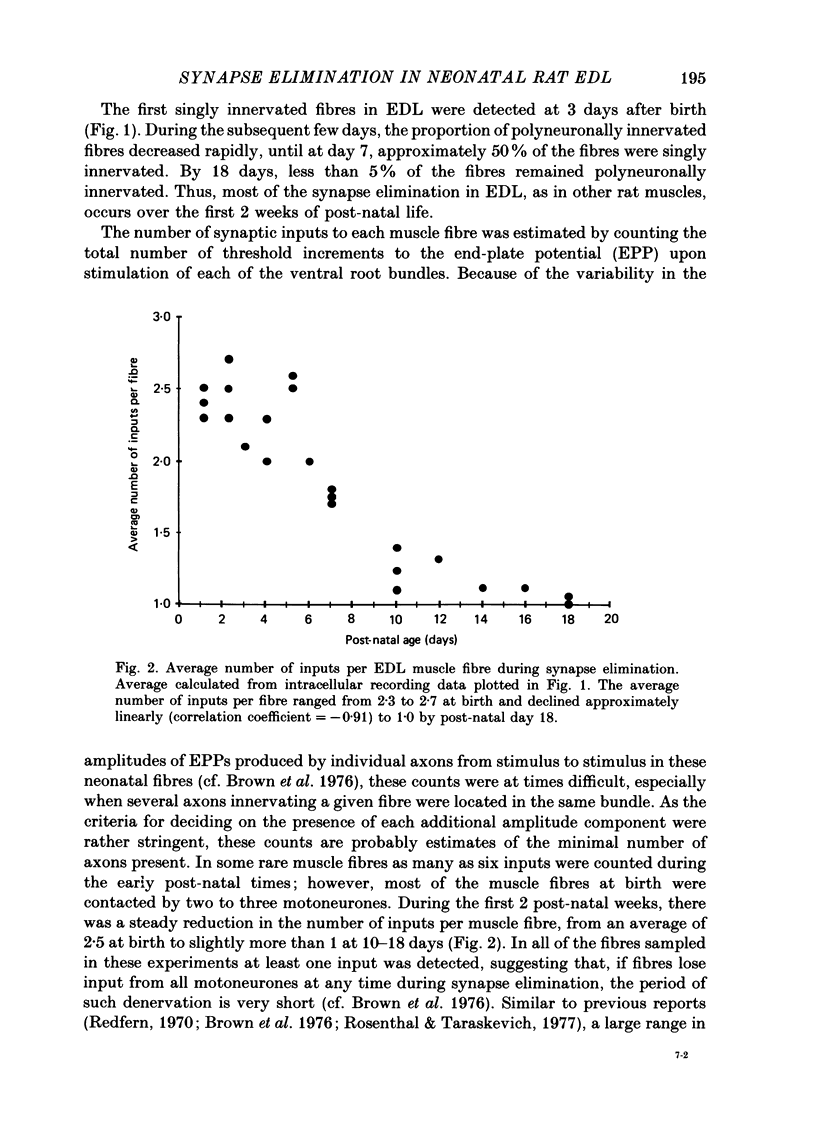
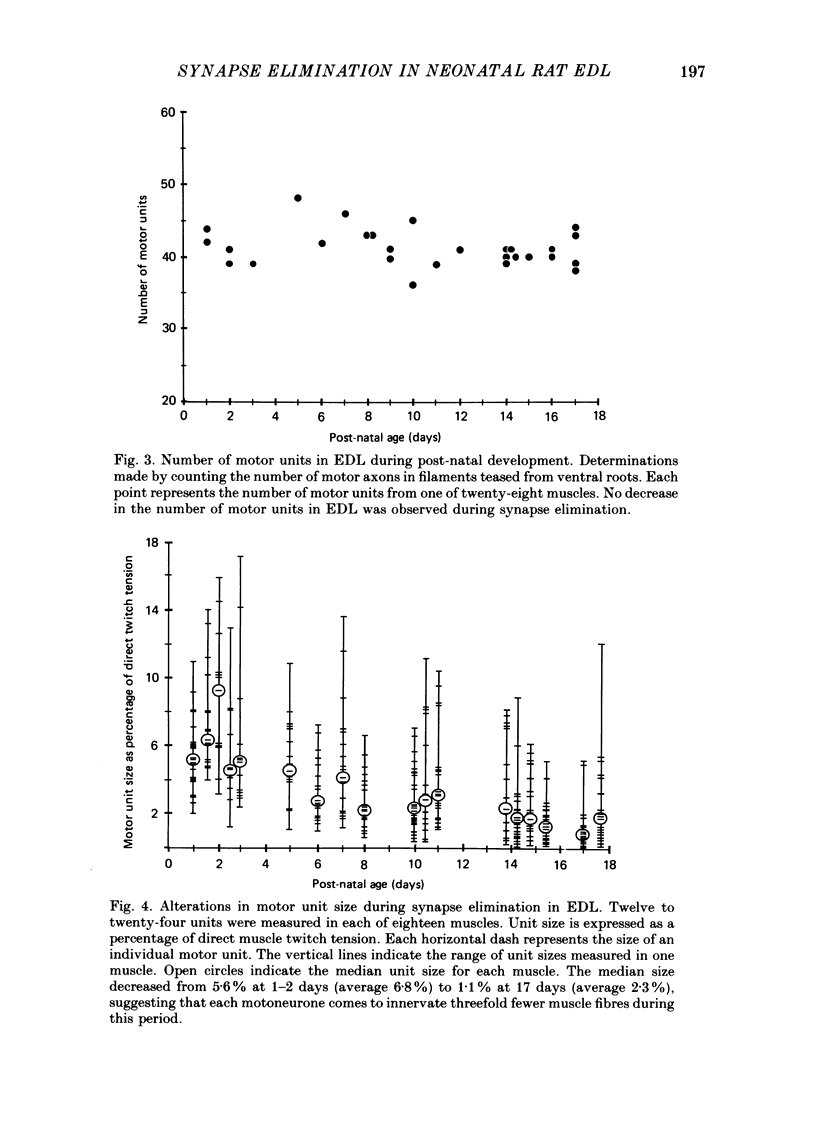
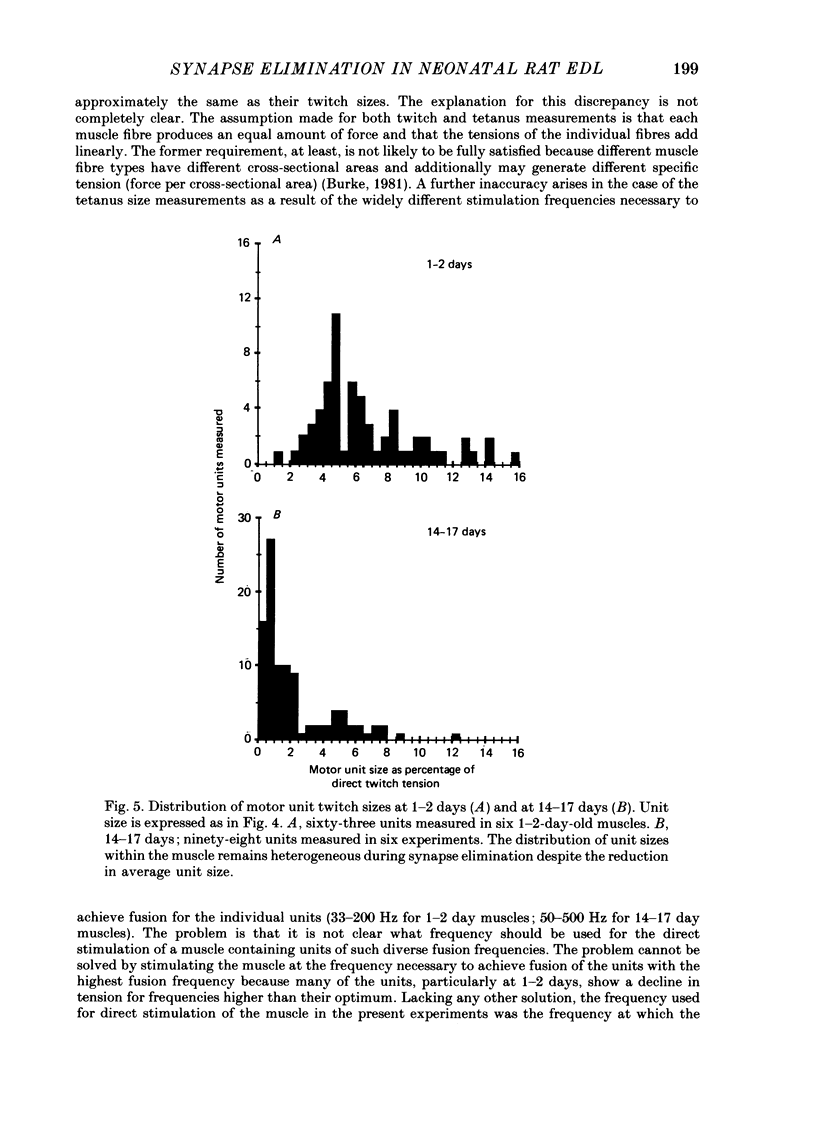
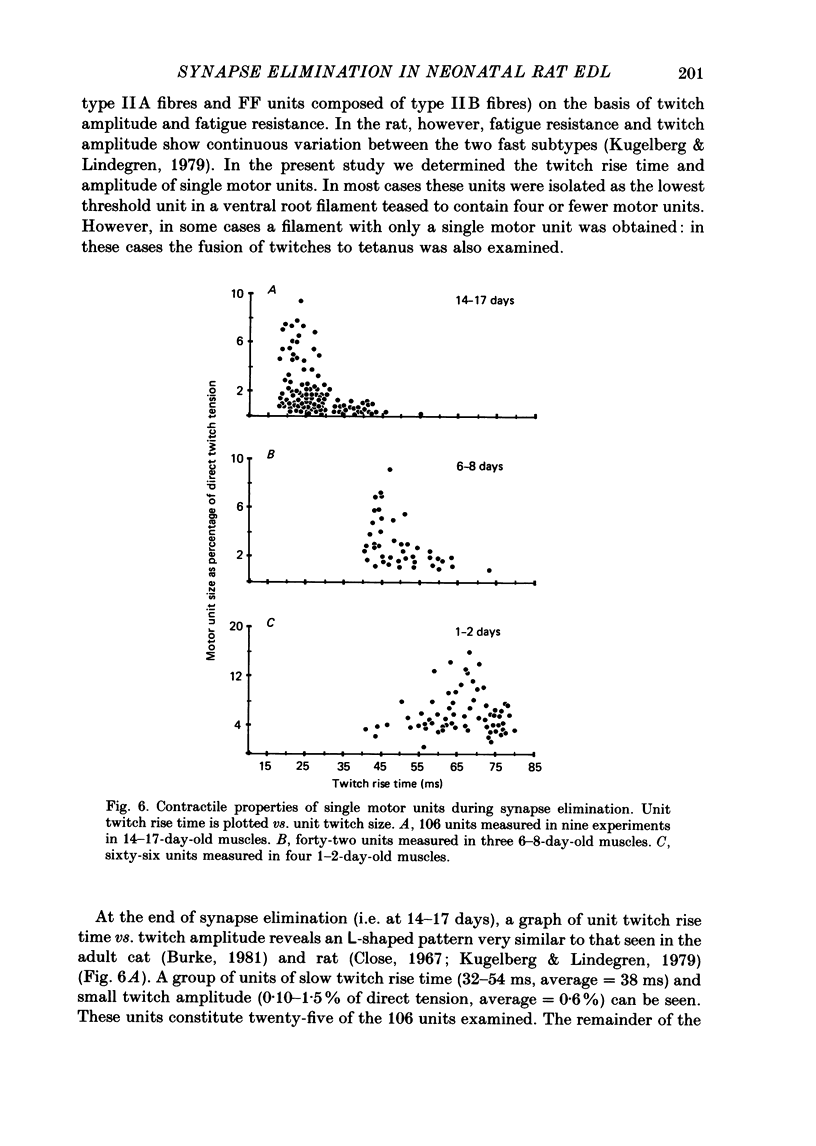
1. We have used in vitro intracellular recordings and measurements of the contractile properties of single motor units to examine the changes in muscle innervation occurring during the post-natal development of a fast-twitch muscle in the hindlimb of the rat, the extensor digitorum longus (EDL). 2. Intracellular recordings of end-plate potentials evoked in response to graded stimulation of the nerve supply to the muscle indicate that during the first day after birth, each muscle fibre receives synaptic input from at least two motoneurones and that some muscle fibres receive as many as six such inputs. With subsequent development, most of this polyneuronal innervation is eliminated: the first singly innervated fibres are encountered on day 3; by day 18 fewer than 5% of the fibres remain polyneuronally innervated. These results show that there are quantitative differences in post-natal synapse elimination in EDL compared to its well-studied counterpart, the soleus. Although the great majority of fibres in both muscles become singly innervated at about 18 days, the first singly innervated fibres appear at least a week earlier in the EDL. None the less, synapses are lost from EDL at about half the rate they are lost from soleus. 3. The number of motor units, determined by counting the number of twitch increments produced by graded stimulation of ventral root filaments teased to contain only a few EDL motor axons, remains unchanged from an average of forty-one from post-natal day 1 to day 17. In addition, the number of muscle fibres counted in muscle cross-sections stained with an anti-myosin antibody increases less than 10% from birth to adulthood. Therefore, synapse elimination in EDL occurs with a largely constant population of muscle fibres as well as motoneurones. 4. Measurements of tensions generated by single motor units indicate that the average size of a motor unit declines from 6.8% of the muscle fibres at day 1 to 2.3% at 17 days. This result indicates that each motoneurone, on average, comes to innervate threefold fewer muscle fibres. Motor units derived from each of the spinal segments innervating the muscle undergo equivalent reductions in motor unit size, indicating that there is no segmental disproportion to synapse elimination in this muscle. At all ages, there is a large diversity of motor unit sizes in the muscle. Synapse elimination therefore appears to maintain rather than decrease this diversity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Westerman R. A. The properties of motor units in a fast and a slow twitch muscle during post-natal development in the kitten. J Physiol. 1974 Feb;237(1):75–90. doi: 10.1113/jphysiol.1974.sp010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon R. J., Thompson W. J. The organization and development of compartmentalized innervation in rat extensor digitorum longus muscle. J Physiol. 1988 Apr;398:211–231. doi: 10.1113/jphysiol.1988.sp017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulac M., Meininger V. Postnatal development and cell death in the sciatic motor nucleus of the mouse. Exp Brain Res. 1983;50(1):107–116. doi: 10.1007/BF00238237. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. Development of the topographical projection of motor neurons to a rat muscle accompanies loss of polyneuronal innervation. J Neurosci. 1984 Sep;4(9):2204–2212. doi: 10.1523/JNEUROSCI.04-09-02204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McGrath P. A., Davey D. F., Hutchinson I. Death of motorneurons during the postnatal loss of polyneuronal innervation of rat muscles. J Comp Neurol. 1983 Aug 10;218(3):351–363. doi: 10.1002/cne.902180311. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Maunsell J. H., Van Essen D. C. Effects of motor unit size on innervation patterns in neonatal mammals. Exp Neurol. 1980 Dec;70(3):516–524. doi: 10.1016/0014-4886(80)90178-8. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., van Essen D. C. Regional differences in the timing of synapse elimination in skeletal muscles of the neonatal rabbit. Brain Res. 1979 Jun 22;169(2):275–286. doi: 10.1016/0006-8993(79)91030-8. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Booth C. M. Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature. 1983 Aug 25;304(5928):741–742. doi: 10.1038/304741a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J., Cosmos E., Brierley J. Differentiation of muscle fiber types in aneurogenic brachial muscles of the chick embryo. J Exp Zool. 1982 Nov 20;224(1):65–80. doi: 10.1002/jez.1402240108. [DOI] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangain J., Vrbová G. Elimination of polyneuronal innervation in a fast muscle of normal and dystrophic mice. J Physiol. 1983 Sep;342:267–275. doi: 10.1113/jphysiol.1983.sp014850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Johnston D. M. Development of a mammalian fast muscle: dynamic and biochemical properties correlated. J Physiol. 1973 Oct;234(1):29–42. doi: 10.1113/jphysiol.1973.sp010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H., Van Essen D. C. Specific innervation of muscle fiber types in a developmentally polyinnervated muscle. Dev Biol. 1985 Sep;111(1):42–50. doi: 10.1016/0012-1606(85)90433-6. [DOI] [PubMed] [Google Scholar]
- Gordon H., van Essen D. C. The relation of neuromuscular synapse elimination to spinal position of rabbit and rat soleus motoneurones. J Physiol. 1983 Jun;339:591–597. doi: 10.1113/jphysiol.1983.sp014736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg C., Kellerth J. O. The postnatal development of some twitch and fatigue properties of single motor units in the ankle muscles of the kitten. Acta Physiol Scand. 1975 Nov;95(3):243–257. doi: 10.1111/j.1748-1716.1975.tb10048.x. [DOI] [PubMed] [Google Scholar]
- Hardman V. J., Brown M. C. Absence of postnatal death among motoneurones supplying the inferior gluteal nerve of the rat. Brain Res. 1985 Mar;351(1):1–9. doi: 10.1016/0165-3806(85)90225-1. [DOI] [PubMed] [Google Scholar]
- Jenq C. B., Chung K., Coggeshall R. E. Postnatal loss of axons in normal rat sciatic nerve. J Comp Neurol. 1986 Feb 22;244(4):445–450. doi: 10.1002/cne.902440404. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Brain Res. 1982 Aug;256(4):473–479. doi: 10.1016/0165-3806(82)90192-4. [DOI] [PubMed] [Google Scholar]
- Lowrie M. B., O'Brien R. A., Vrbová G. The effect of altered peripheral field on motoneurone function in developing rat soleus muscles. J Physiol. 1985 Nov;368:513–524. doi: 10.1113/jphysiol.1985.sp015873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y., Yoshioka K. Selective elimination of motor nerve terminals in the rat soleus muscle during development. J Physiol. 1980 Dec;309:631–646. doi: 10.1113/jphysiol.1980.sp013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe V., NcGrath P. A., Bennett M. R. Postnatal death of motor neurons during the development of the brachial spinal cord of the rat. Neurosci Lett. 1981 Dec 23;27(3):249–254. doi: 10.1016/0304-3940(81)90438-9. [DOI] [PubMed] [Google Scholar]
- Ontell M., Dunn R. F. Neonatal muscle growth: a quantitative study. Am J Anat. 1978 Aug;152(4):539–555. doi: 10.1002/aja.1001520408. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. The absence of significant postnatal motoneuron death in the brachial and lumbar spinal cord of the rat. J Comp Neurol. 1986 Apr 8;246(2):281–286. doi: 10.1002/cne.902460211. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A non-phosphate-buffered physiological saline for in vitro electrophysiological studies on the mammalian neuromuscular junction [proceedings]. J Physiol. 1978 May;278:8P–9P. [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity and myosin heavy chains of developing rabbit muscle fibers. J Biol Chem. 1985 Nov 25;260(27):14403–14405. [PubMed] [Google Scholar]
- Rosenthal J. L., Taraskevich P. S. Reduction of multiaxonal innervation at the neuromuscular junction of the rat during development. J Physiol. 1977 Sep;270(2):299–310. doi: 10.1113/jphysiol.1977.sp011953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Development of muscle fiber specialization in the rat hindlimb. J Cell Biol. 1981 Jul;90(1):128–144. doi: 10.1083/jcb.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C. R. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev Biol. 1982 Nov;94(1):11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Taxt T., Ding R., Jansen J. K. A note on the elimination of polyneuronal innervation of skeletal muscles in neonatal rats. Acta Physiol Scand. 1983 Apr;117(4):557–560. doi: 10.1111/j.1748-1716.1983.tb07226.x. [DOI] [PubMed] [Google Scholar]
- Thompson W. J. Activity and synapse elimination at the neuromuscular junction. Cell Mol Neurobiol. 1985 Jun;5(1-2):167–182. doi: 10.1007/BF00711091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J. Lack of segmental selectivity in elimination of synapses from soleus muscle of new-born rats. J Physiol. 1983 Feb;335:343–352. doi: 10.1113/jphysiol.1983.sp014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Sutton L. A., Riley D. A. Fibre type composition of single motor units during synapse elimination in neonatal rat soleus muscle. Nature. 1984 Jun 21;309(5970):709–711. doi: 10.1038/309709a0. [DOI] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]


