Abstract
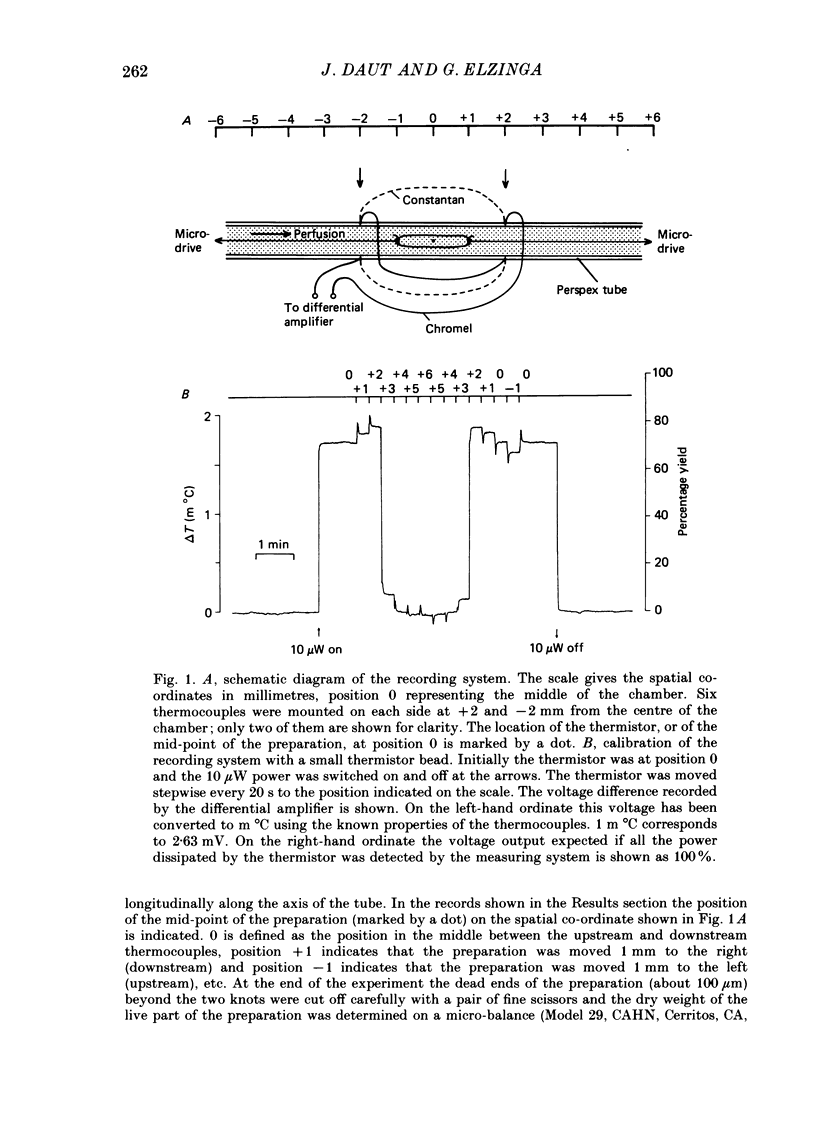
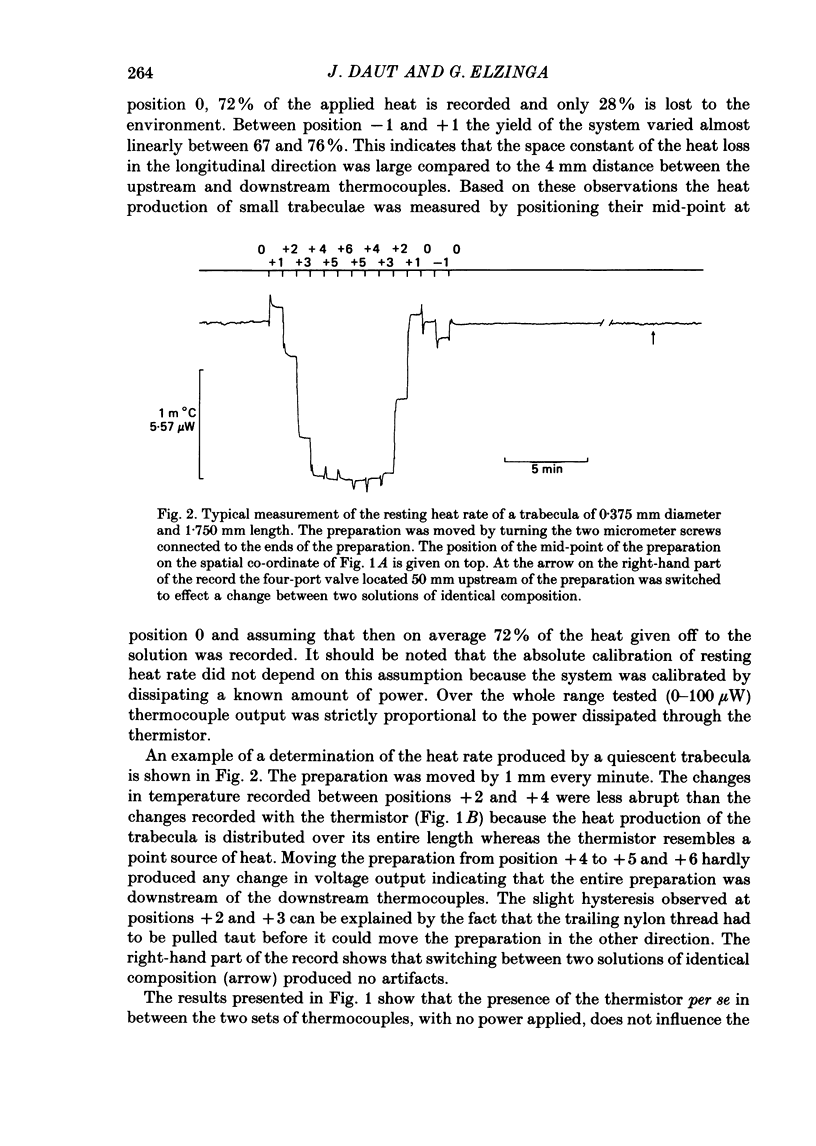
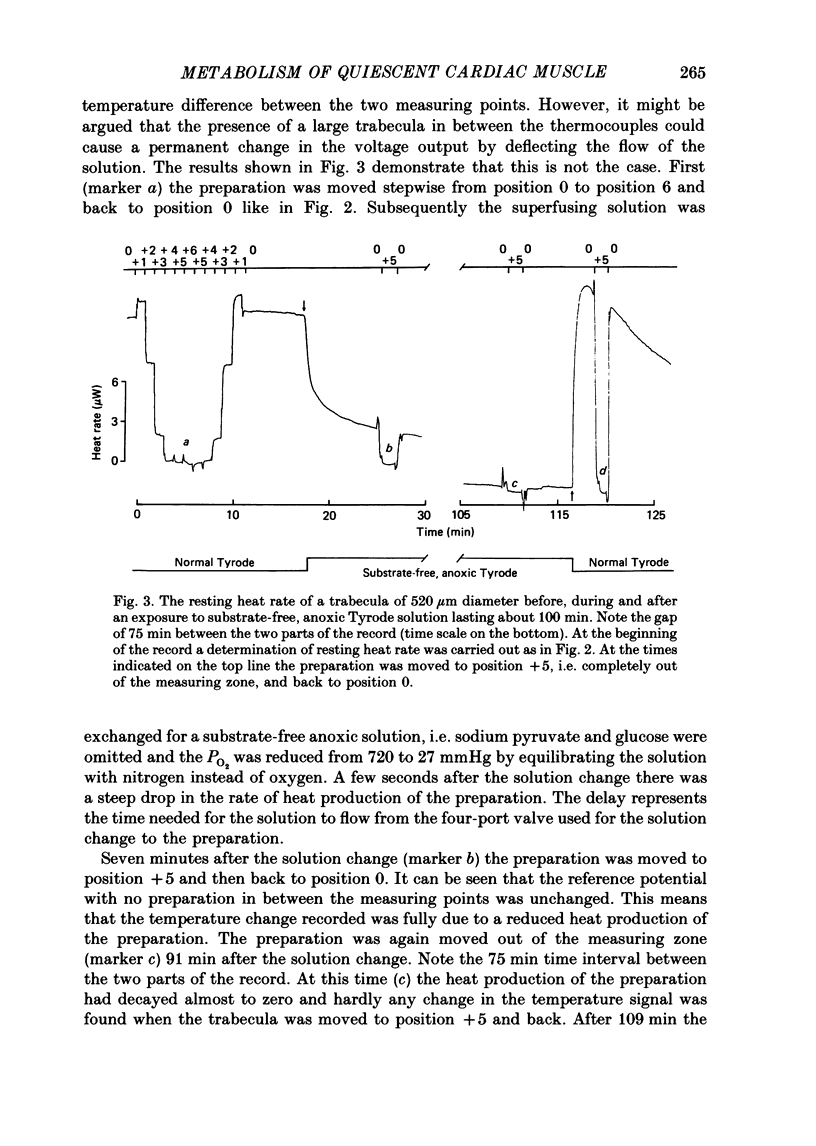
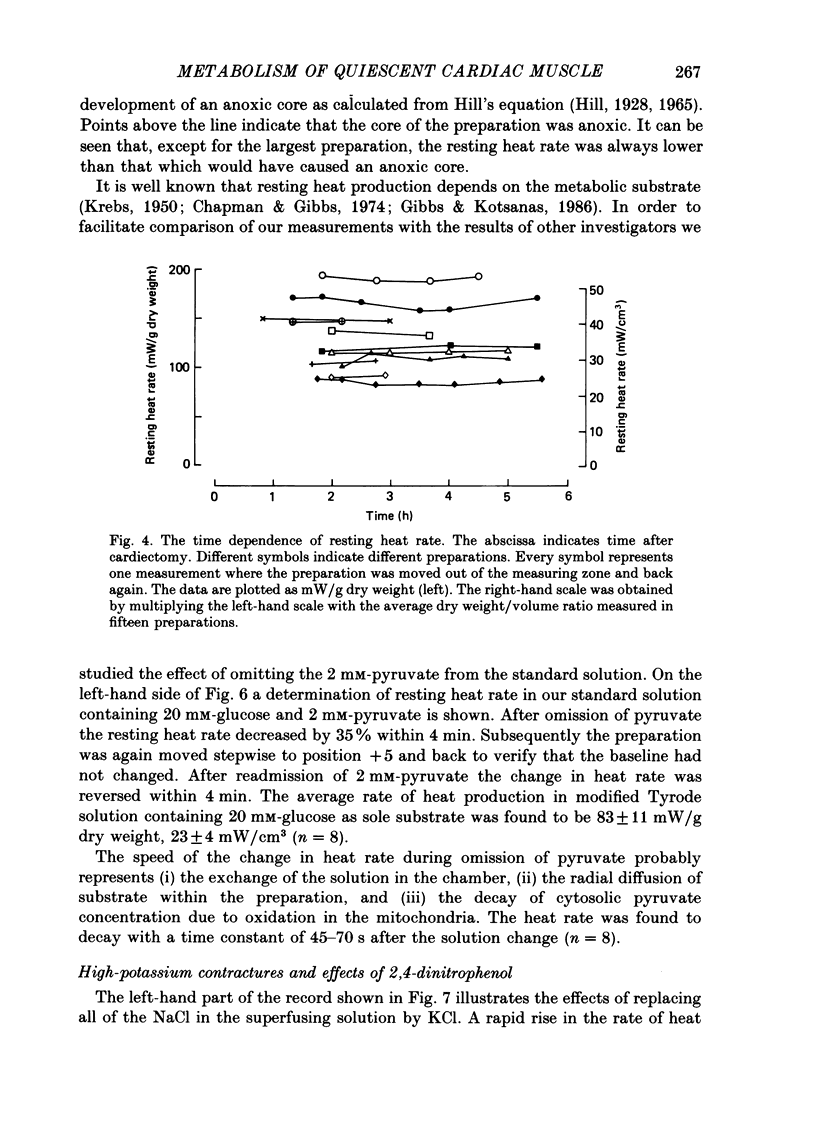
1. A new calorimetric technique has been developed which allows continuous measurement of the rate of energy expenditure in superfused preparations of cardiac muscle. Thin trabeculae of guinea-pig ventricular muscle were mounted in a Perspex tube of 0.8 mm inner diameter and the temperature difference of the perfusate upstream and downstream of the preparation was measured. 2. The resting heat rate of trabeculae of 240-575 microns diameter from guinea-pig heart was determined repeatedly for up to 6 h after cardiectomy. It did not vary with time during the course of the experiment. 3. The average resting heat rate measured in HEPES-buffered Tyrode solution containing 20 mM-glucose and 2 mM-pyruvate as substrates was 130 +/- 29 mW/g dry weight or 36 +/- 8 mW/cm3 of tissue (n = 15). This is an order of magnitude larger than the resting heat rate reported in the literature for isolated cardiac preparations. 4. After omitting the pyruvate from the superfusate the resting heat rate decreased to 60-70% of its steady value within 4 min. After readmission of pyruvate this effect was reversed. The average resting heat rate with glucose as sole substrate was 23 +/- 4 mW/cm3. 5. Uncoupling of the mitochondria by 50 microM-2,4-dinitrophenol (DNP) increased the heat rate up to 170 mW/cm3. This effect could be maintained for several minutes and was fully reversible. Raising the external K+ concentration to 150 mM (NaCl replaced by KCl) induced a transient rise in the rate of heat production up to 115 mW/cm3. 6. The heat production during uncoupling of the mitochondria and during potassium contractures was inversely related to the diameter of the preparation. Calculation based on Hill's equation (Hill, 1928) indicated that this was caused by the development of anoxia at the core of the preparation. 7. In contrast, the rate of heat production of quiescent preparations was not correlated with diameter and calculation indicated that at rest there was no anoxic core. The high value of resting heat rate found in the present study is discussed within the context of the large variation of 1.7-25 mW/g reported in the literature for resting metabolic rate of cardiac muscle.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
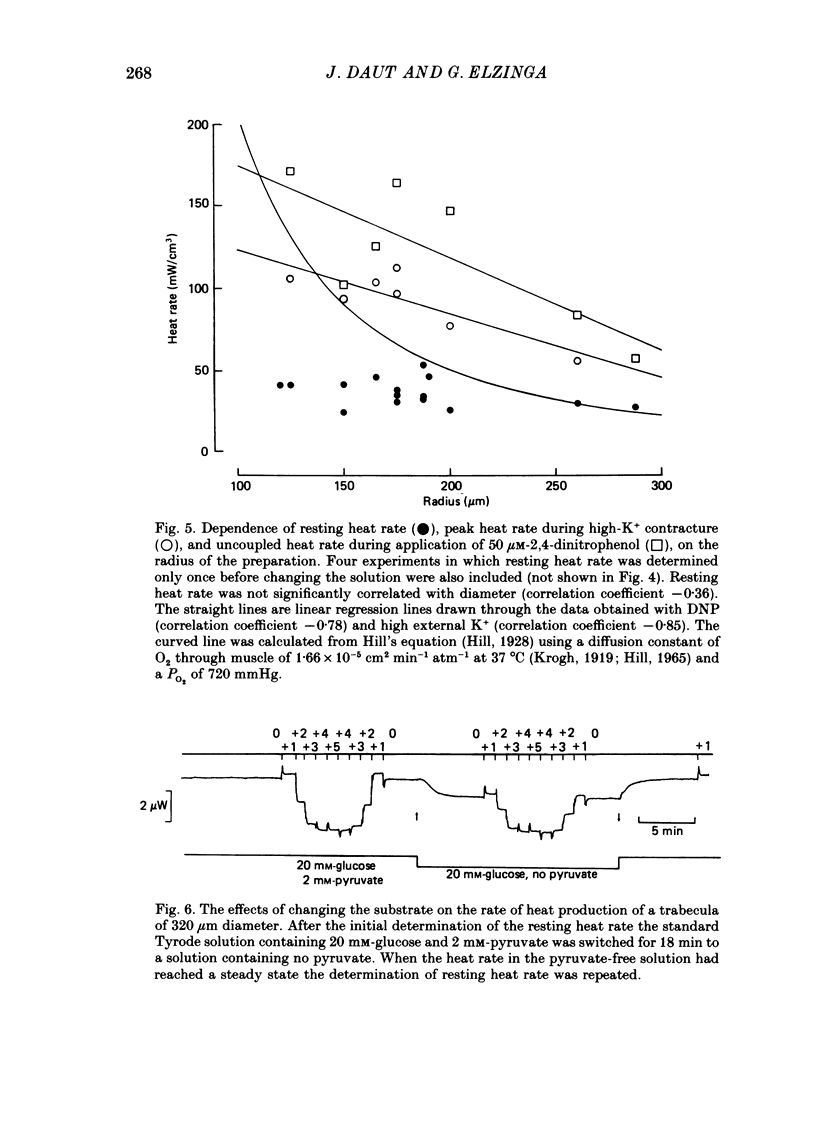
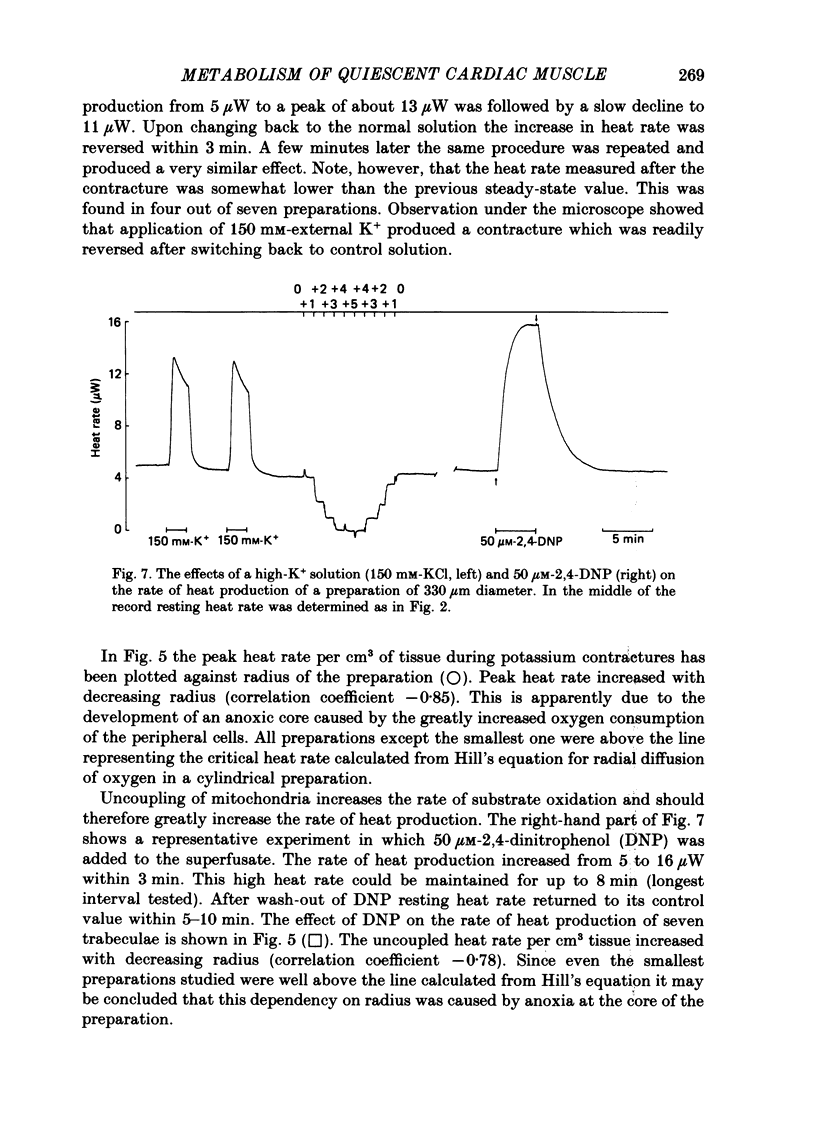
- Bardenheuer H., Schrader J. Relationship between myocardial oxygen consumption, coronary flow, and adenosine release in an improved isolated working heart preparation of guinea pigs. Circ Res. 1983 Mar;52(3):263–271. doi: 10.1161/01.res.52.3.263. [DOI] [PubMed] [Google Scholar]
- Bünger R., Permanetter B. Parallel stimulation by Ca2+ of inotropism and pyruvate dehydrogenase in perfused heart. Am J Physiol. 1984 Jul;247(1 Pt 1):C45–C52. doi: 10.1152/ajpcell.1984.247.1.C45. [DOI] [PubMed] [Google Scholar]
- Bünger R., Permanetter B., Sommer O., Yaffe S. Adaptive changes of pyruvate oxidation in perfused heart during adrenergic stimulation. Am J Physiol. 1982 Jan;242(1):H30–H36. doi: 10.1152/ajpheart.1982.242.1.H30. [DOI] [PubMed] [Google Scholar]
- CRANEFIELD P. F., GREENSPAN K. The rate of oxygen uptake of quiescent cardiac muscle. J Gen Physiol. 1960 Nov;44:235–249. doi: 10.1085/jgp.44.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. B., Gibbs C. L. The effect of metabolic substrate on mechanical activity and heart production in papillary muscle. Cardiovasc Res. 1974 Sep;8(5):656–667. doi: 10.1093/cvr/8.5.656. [DOI] [PubMed] [Google Scholar]
- Coulson R. L. Energetics of isovolumic contractions of the isolated rabbit heart. J Physiol. 1976 Aug;260(1):45–53. doi: 10.1113/jphysiol.1976.sp011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. High potassium and low sodium contractures in sheep cardiac muscle. J Gen Physiol. 1971 Nov;58(5):483–510. doi: 10.1085/jgp.58.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. L. Cardiac energetics. Physiol Rev. 1978 Jan;58(1):174–254. doi: 10.1152/physrev.1978.58.1.174. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Kotsanas G. Factors regulating basal metabolism of the isolated perfused rabbit heart. Am J Physiol. 1986 Jun;250(6 Pt 2):H998–1007. doi: 10.1152/ajpheart.1986.250.6.H998. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Papadoyannis D. E., Drake A. J., Noble M. I. Oxygen consumption of the nonworking and potassium chloride-arrested dog heart. Circ Res. 1980 Sep;47(3):408–417. doi: 10.1161/01.res.47.3.408. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Woolley G., Kotsanas G., Gibson W. R. Cardiac energetics in daunorubicin-induced cardiomyopathy. J Mol Cell Cardiol. 1984 Oct;16(10):953–962. doi: 10.1016/s0022-2828(84)80031-0. [DOI] [PubMed] [Google Scholar]
- Holubarsch C., Alpert N. R., Goulette R., Mulieri L. A. Heat production during hypoxic contracture of rat myocardium. Circ Res. 1982 Dec;51(6):777–786. doi: 10.1161/01.res.51.6.777. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Kennedy F. G., Jones D. P. Oxygen dependence of mitochondrial function in isolated rat cardiac myocytes. Am J Physiol. 1986 Mar;250(3 Pt 1):C374–C383. doi: 10.1152/ajpcell.1986.250.3.C374. [DOI] [PubMed] [Google Scholar]
- Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol. 1919 May 20;52(6):391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K. S. The relationship of the oxygen consumption to the contraction of the cat papillary muscle. J Physiol. 1960 Apr;151:186–201. [PMC free article] [PubMed] [Google Scholar]
- Lochner W., Arnold G., Müller-Ruchholtz E. R. Metabolism of the artificially arrested heart and of the gas-perfused heart. Am J Cardiol. 1968 Sep;22(3):299–311. doi: 10.1016/0002-9149(68)90114-8. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S., Gibbs C. L. Factors affecting the metabolism of resting rabbit papillary muscle. Pflugers Arch. 1983 Mar;396(4):285–291. doi: 10.1007/BF01063932. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S., Gibbs C. L. Species differences in cardiac energetics. Am J Physiol. 1979 Jul;237(1):H90–H98. doi: 10.1152/ajpheart.1979.237.1.H90. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S. The effect of temperature on the basal metabolism of cardiac muscle. Pflugers Arch. 1985 Sep;405(2):163–169. doi: 10.1007/BF00584538. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S. The rate of resting heat production of rat papillary muscle. Pflugers Arch. 1985 Sep;405(2):155–162. doi: 10.1007/BF00584537. [DOI] [PubMed] [Google Scholar]
- Montini J., Bagby G. J., Spitzer J. J. Importance of exogenous substrates for the energy production of adult rat heart myocytes. J Mol Cell Cardiol. 1981 Oct;13(10):903–911. doi: 10.1016/0022-2828(81)90289-3. [DOI] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Rose H., Kammermeier H. Contraction and metabolic activity of electrically stimulated cardiac myocytes from adult rats. Pflugers Arch. 1986 Jul;407(1):116–118. doi: 10.1007/BF00580731. [DOI] [PubMed] [Google Scholar]
- Rouleau J. L., Paradis P., Shenasa H., Juneau C. Faster time to peak tension and velocity of shortening in right versus left ventricular trabeculae and papillary muscles of dogs. Circ Res. 1986 Nov;59(5):556–561. doi: 10.1161/01.res.59.5.556. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Uglesity A. Na+-Ca2+ exchange contributes to increase of cytosolic Ca2+ concentration during depolarization in heart muscle. Am J Physiol. 1986 Apr;250(4 Pt 1):C651–C656. doi: 10.1152/ajpcell.1986.250.4.C651. [DOI] [PubMed] [Google Scholar]
- Spieckermann P. G., Piper H. M. Oxygen demand of calcium-tolerant adult cardiac myocytes. Basic Res Cardiol. 1985;80 (Suppl 2):71–74. [PubMed] [Google Scholar]
- WHALEN W. J. Some factors influencing O2 consumption of isolated heart muscle. Am J Physiol. 1960 Jun;198:1153–1156. doi: 10.1152/ajplegacy.1960.198.6.1153. [DOI] [PubMed] [Google Scholar]


