Abstract
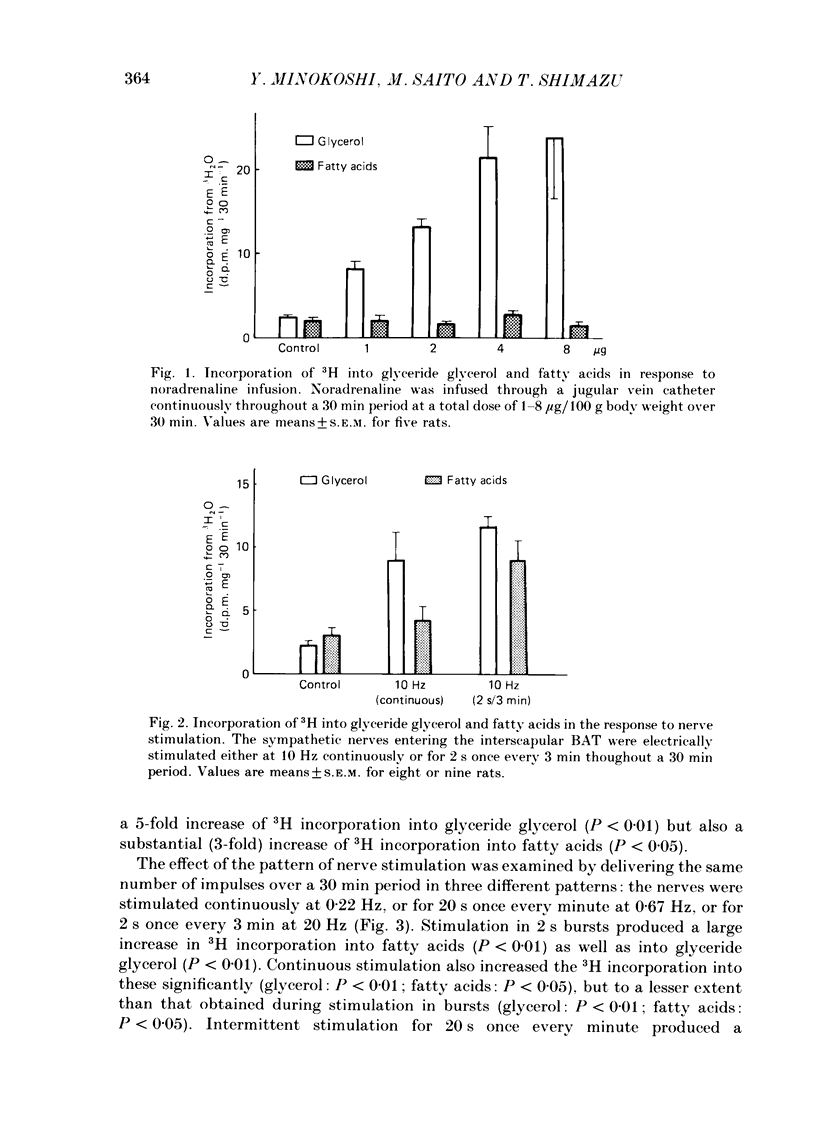
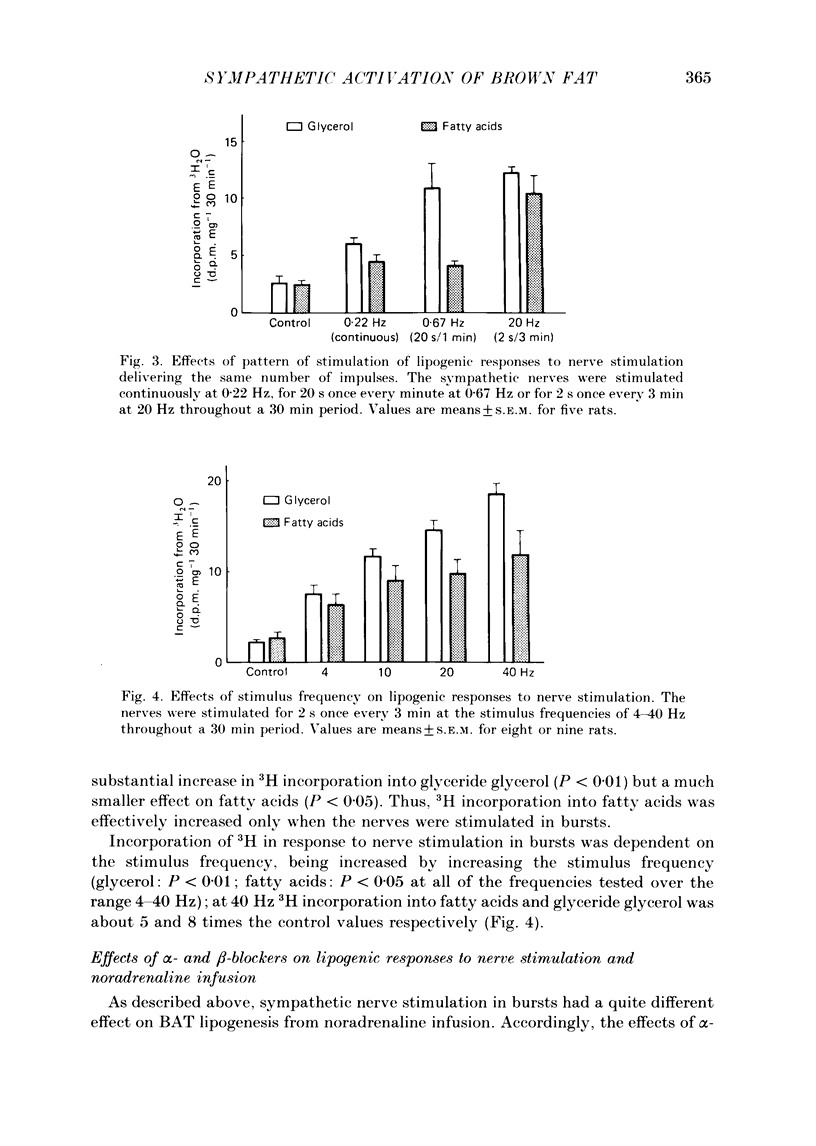
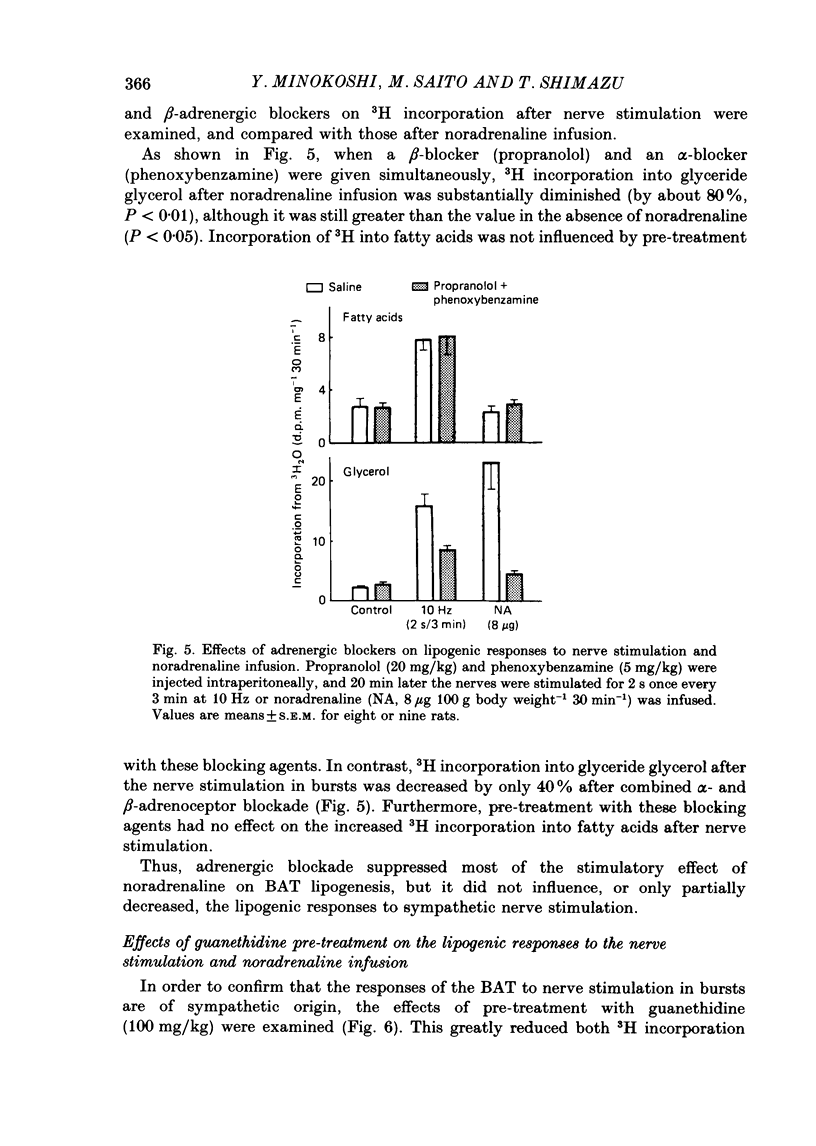
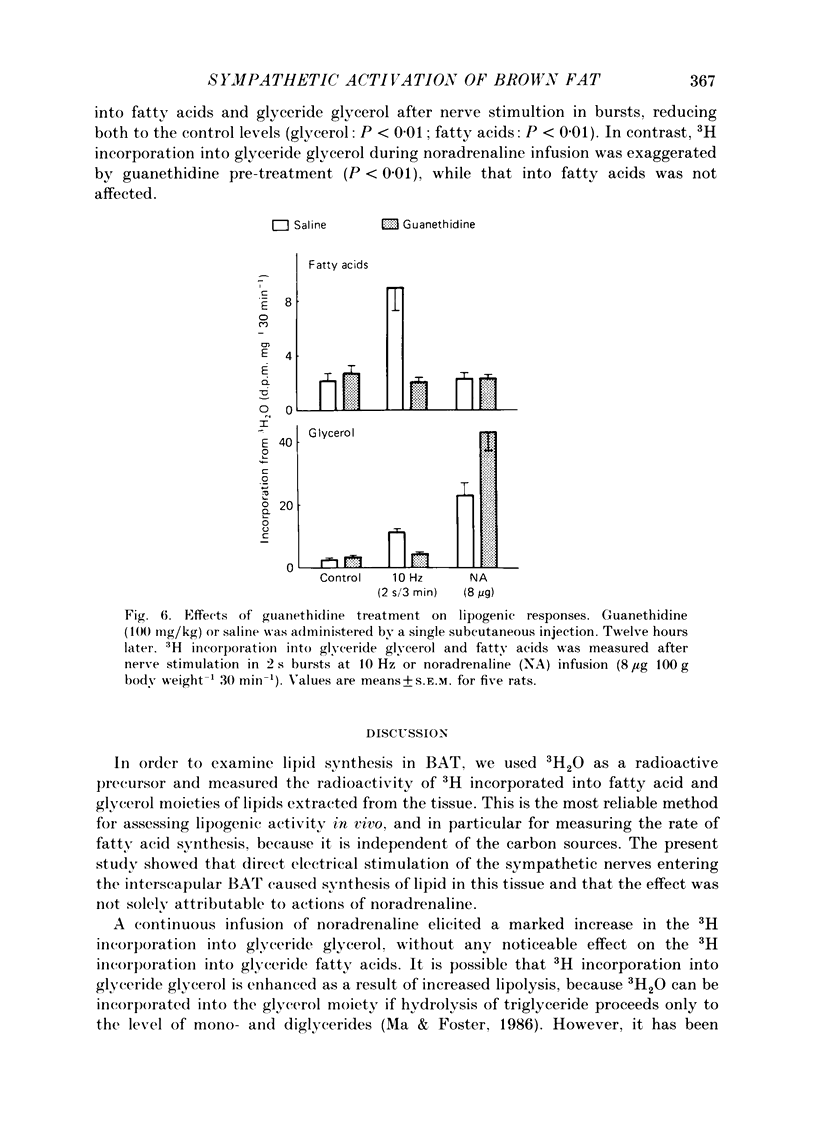
1. The roles of the sympathetic nerves in regulating lipid synthesis in brown adipose tissue (BAT) were studied by measuring incorporation of 3H from 3H2O into glyceride glycerol and glyceride fatty acids in the interscapular BAT in anaesthetized rats. 2. When noradrenaline was infused intravenously at a total dose of 1-8 micrograms/100 g body weight over 30 min, 3H incorporation into glyceride glycerol increased whereas 3H incorporation into fatty acids did not change. Similar responses were found when the sympathetic nerves entering the interscapular BAT were stimulated continuously at 10 Hz. However, when electrical stimuli consisting of a much shorter train (2 s) were applied to the nerves at 3 min intervals at 10 Hz (stimulation in bursts). 3H incorporation into both glyceride glycerol and fatty acids was enhanced. Stimulation in bursts elicited more pronounced lipogenic responses than other patterns that were employed, and involved the delivery of precisely the same number of impulses over the whole period of stimulation. The lipogenic responses to nerve stimulation in bursts were increased by increasing the stimulus frequency over the range 4-40 Hz. 3. Simultaneous administration of propranolol and phenoxybenzamine had little effect on either the fatty acid or the glyceride glycerol response to nerve stimulation. In contrast, these blocking agents almost completely eliminated the responses to noradrenaline infusion. 4. Pre-treatment with guanethidine effectively abolished the lipogenic response to nerve stimulation but potentiated the response to noradrenaline infusion. 5. It is concluded that lipid synthesis in BAT is enhanced by direct electrical stimulation of the sympathetic nerves only when they are stimulated in bursts. Sympathetic activation of lipogenesis in BAT is not solely attributable to the action of noradrenaline but involves some non-adrenergic mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Bircham P. M., Bloom S. R., Edwards A. V. Release of neuropeptide Y in response to splanchnic nerve stimulation in the conscious calf. J Physiol. 1984 Dec;357:401–408. doi: 10.1113/jphysiol.1984.sp015507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P. O., Bloom S. R., Edwards A. V., Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982 Jan;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P. O., Bloom S. R., Järhult J. Colonic motor and vascular responses to pelvic nerve stimulation and their relation to local peptide release in the cat. J Physiol. 1983 Jan;334:293–307. doi: 10.1113/jphysiol.1983.sp014495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J., Lundberg J. M., Hökfelt T., Terenius L., Goldstein M. 'Neuropeptide tyrosine' (NPY) is co-stored with noradrenaline in vascular but not in parenchymal sympathetic nerves of brown adipose tissue. Exp Cell Res. 1986 Jun;164(2):546–550. doi: 10.1016/0014-4827(86)90052-2. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Caterson I. D., Newsholme E. A. The effect of insulin and noradrenaline on the uptake of 2-[1-14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett. 1985 Sep 2;188(2):257–261. doi: 10.1016/0014-5793(85)80383-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978 Feb;56(1):110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol. 1981;313:351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins J. M., Denton R. M., McCormack J. G. Evidence that noradrenaline increases pyruvate dehydrogenase activity and decreases acetyl-CoA carboxylase activity in rat interscapular brown adipose tissue in vivo. Biochem J. 1985 Jun 15;228(3):751–755. doi: 10.1042/bj2280751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. L., Myant N. B. A comparison between the effects of cold exposure in vivo and of noradrenaline in vitro on the metabolism of the brown fat of new-born rabbits. Biochem J. 1970 Aug;119(1):103–111. doi: 10.1042/bj1190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Theodorsson-Norheim E., Pernow J. Guanethidine-sensitive release of neuropeptide Y-like immunoreactivity in the cat spleen by sympathetic nerve stimulation. Neurosci Lett. 1984 Nov 23;52(1-2):175–180. doi: 10.1016/0304-3940(84)90370-7. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T. Multiple co-existence of peptides and classical transmitters in peripheral autonomic and sensory neurons--functional and pharmacological implications. Prog Brain Res. 1986;68:241–262. doi: 10.1016/s0079-6123(08)60242-3. [DOI] [PubMed] [Google Scholar]
- Ma S. W., Foster D. O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol. 1986 May;64(5):609–614. doi: 10.1139/y86-101. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y., Saito M., Shimazu T. Sympathetic denervation impairs responses of brown adipose tissue to VMH stimulation. Am J Physiol. 1986 Nov;251(5 Pt 2):R1005–R1008. doi: 10.1152/ajpregu.1986.251.5.R1005. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Rothwell N. J., Stock M. J., Stone T. W. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981 Jan 29;289(5796):401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. Influence of noradrenaline on blood flow to brown adipose tissue in rats exhibiting diet-induced thermogenesis. Pflugers Arch. 1981 Mar;389(3):237–242. doi: 10.1007/BF00584784. [DOI] [PubMed] [Google Scholar]
- Saito M., Minokoshi Y., Shimazu T. Brown adipose tissue after ventromedial hypothalamic lesions in rats. Am J Physiol. 1985 Jan;248(1 Pt 1):E20–E25. doi: 10.1152/ajpendo.1985.248.1.E20. [DOI] [PubMed] [Google Scholar]
- Saito M., Minokoshi Y., Shimazu T. Ventromedial hypothalamic stimulation accelerates norepinephrine turnover in brown adipose tissue of rats. Life Sci. 1987 Jul 13;41(2):193–197. doi: 10.1016/0024-3205(87)90493-0. [DOI] [PubMed] [Google Scholar]
- Shimazu T., Takahashi A. Stimulation of hypothalamic nuclei has differential effects on lipid synthesis in brown and white adipose tissue. Nature. 1980 Mar 6;284(5751):62–63. doi: 10.1038/284062a0. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Shimazu T. Hypothalamic regulation of lipid metabolism in the rat: effect of hypothalamic stimulation on lipogenesis. J Auton Nerv Syst. 1982 Sep;6(2):225–235. doi: 10.1016/0165-1838(82)90053-4. [DOI] [PubMed] [Google Scholar]


