Abstract
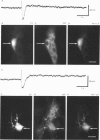
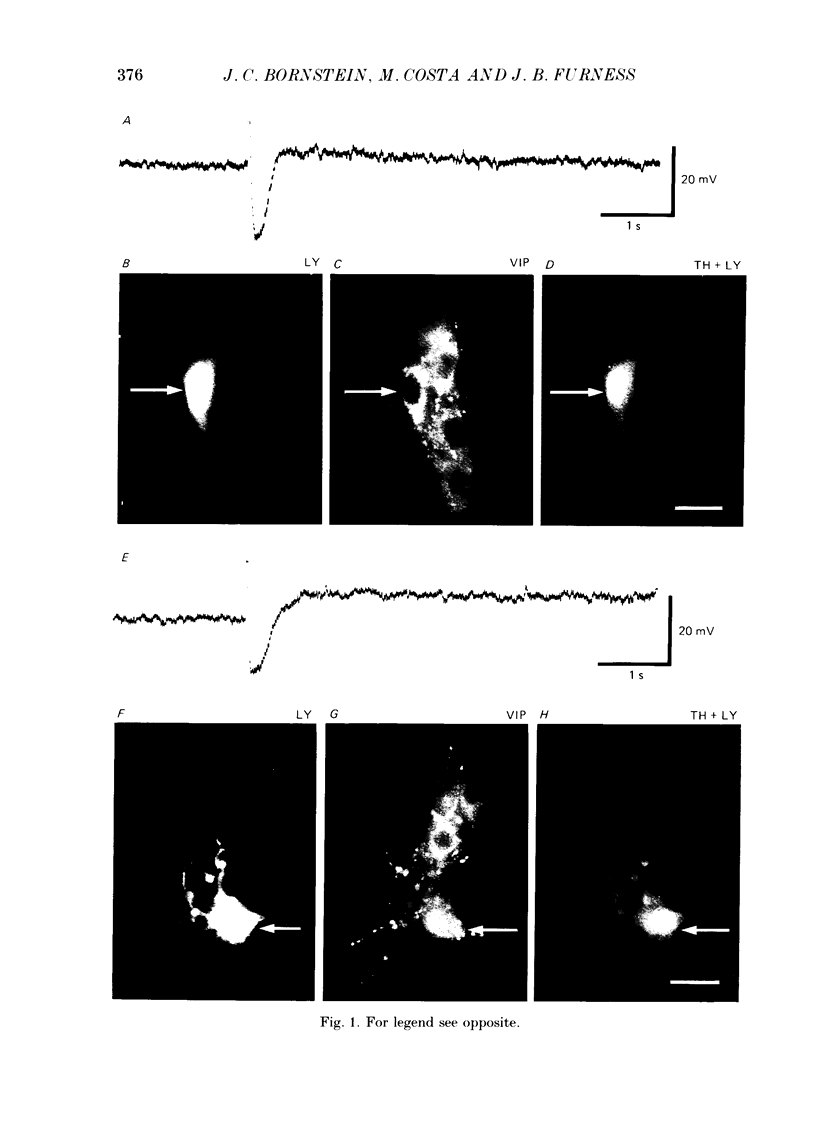
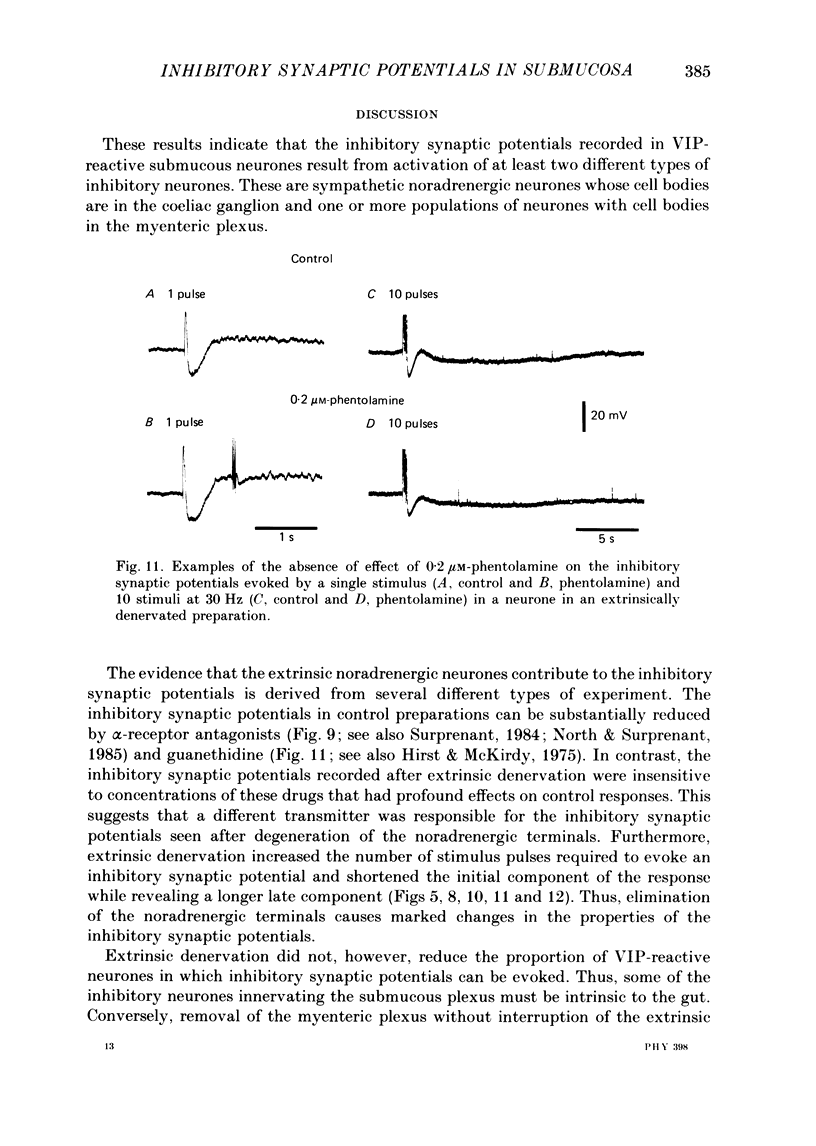
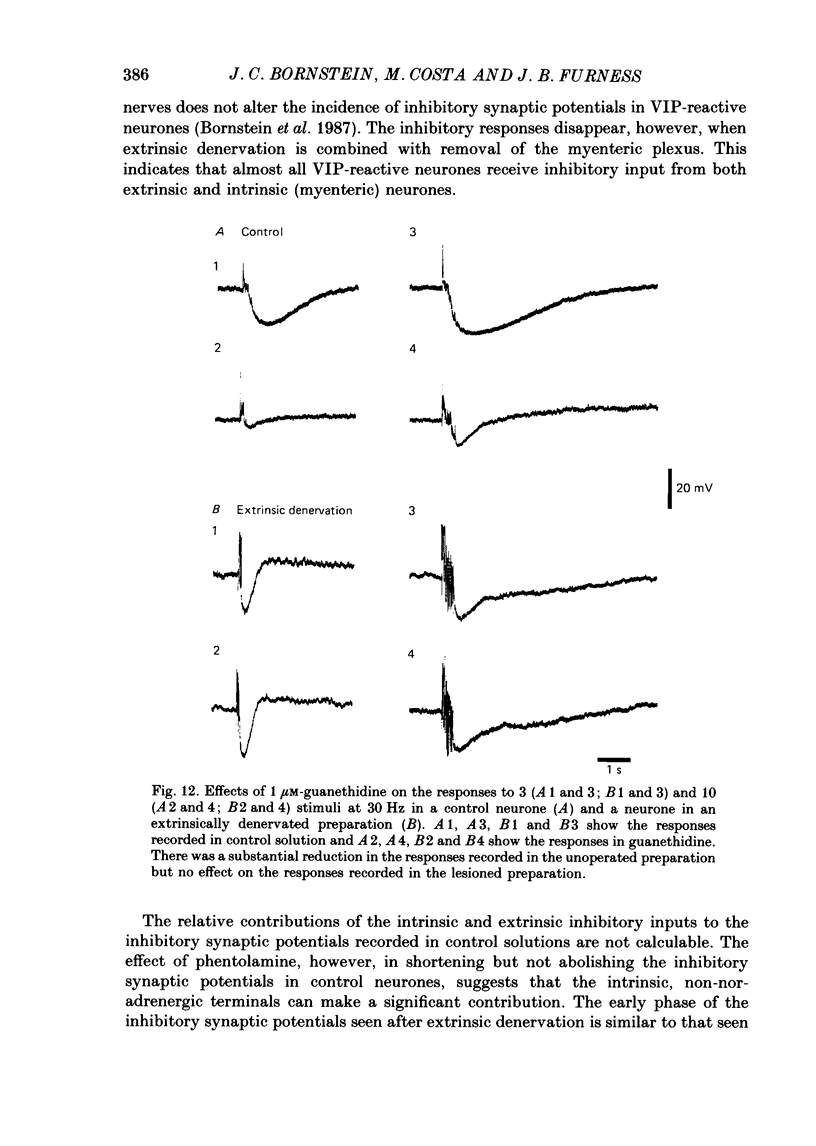
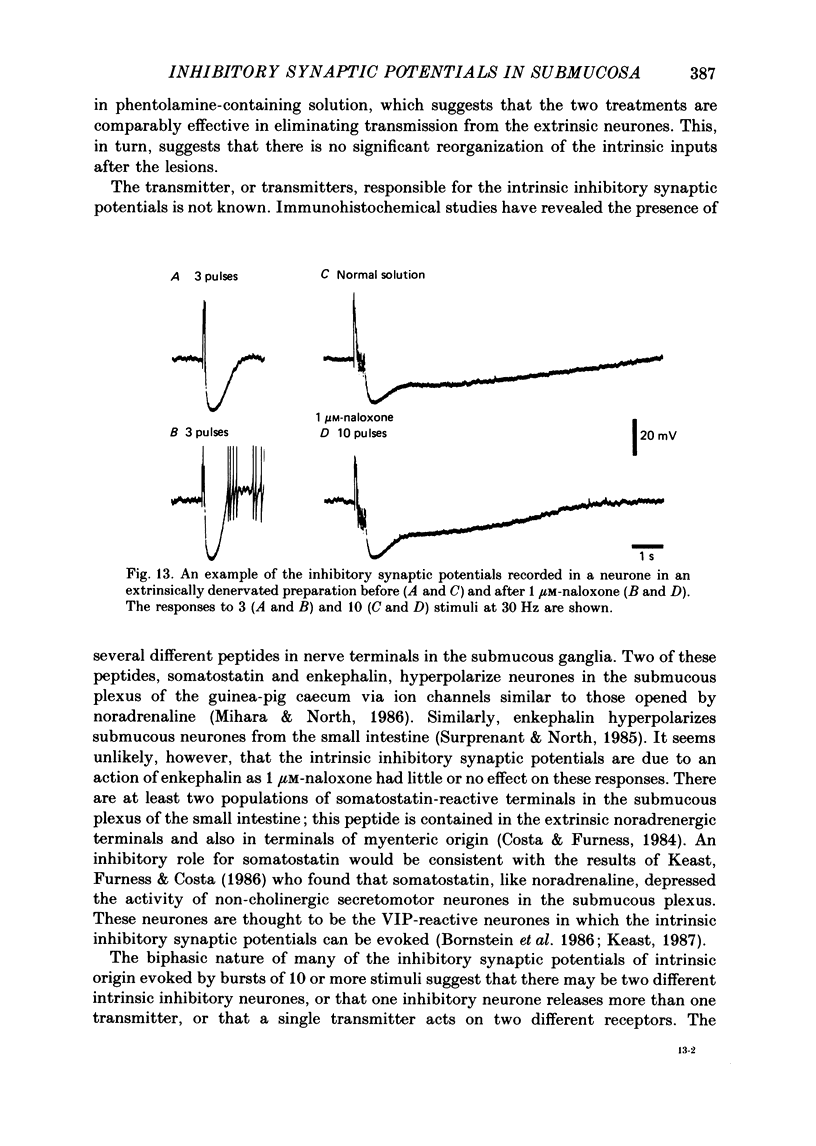
1. The sources of inhibitory synaptic inputs to neurones in submucous ganglia of the guinea-pig small intestine were examined by making lesions to cause selective degeneration of nerve terminals of sympathetic or intrinsic origin. Intracellular recordings were used to evaluate the effects of lesions on the inhibitory inputs. Immunohistochemical techniques were used to identify the neurochemical classes of the impaled neurones and to confirm the efficacy of the lesions. 2. The neurones from which recordings were taken were filled with the fluorescent dye Lucifer Yellow. The preparations were then fixed and processed for immunohistochemistry. 3. Thirty-one neurones reactive for vasoactive intestinal polypeptide (VIP) were examined in control submucous ganglia and all exhibited inhibitory synaptic potentials. In preparations extrinsically denervated by severing the mesenteric nerves, twenty-seven of twenty-eight VIP-reactive neurones had inhibitory synaptic potentials. This indicates that these neurones receive inhibitory synaptic inputs from intrinsic neurones. However, significantly more stimuli were required to evoke a detectable inhibitory synaptic potential in extrinsically denervated preparations than in normal intestine. 4. Extrinsic denervations were combined with removal of the myenteric plexus so that nerve terminals arising from both cell bodies in extrinsic ganglia and in the myenteric plexus degenerated. Under these conditions no inhibitory synaptic potentials could be recorded in any of the nine VIP-reactive neurones studied. 5. The conductance change underlying the intrinsic inhibitory synaptic potentials appeared to be similar to that underlying the responses in normal intestine. 6. The time courses of the intrinsic inhibitory synaptic potentials differed from those of the control responses. The responses to short trains of stimuli were significantly briefer and the responses to long trains significantly more prolonged in the extrinsically denervated preparations than in normal preparations. 7. The intrinsic inhibitory synaptic potentials were not significantly affected by phentolamine (0.2 microM), guanethidine (1 microM) or naloxone (1 microM), although the first two drugs markedly depressed control inhibitory synaptic potentials.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




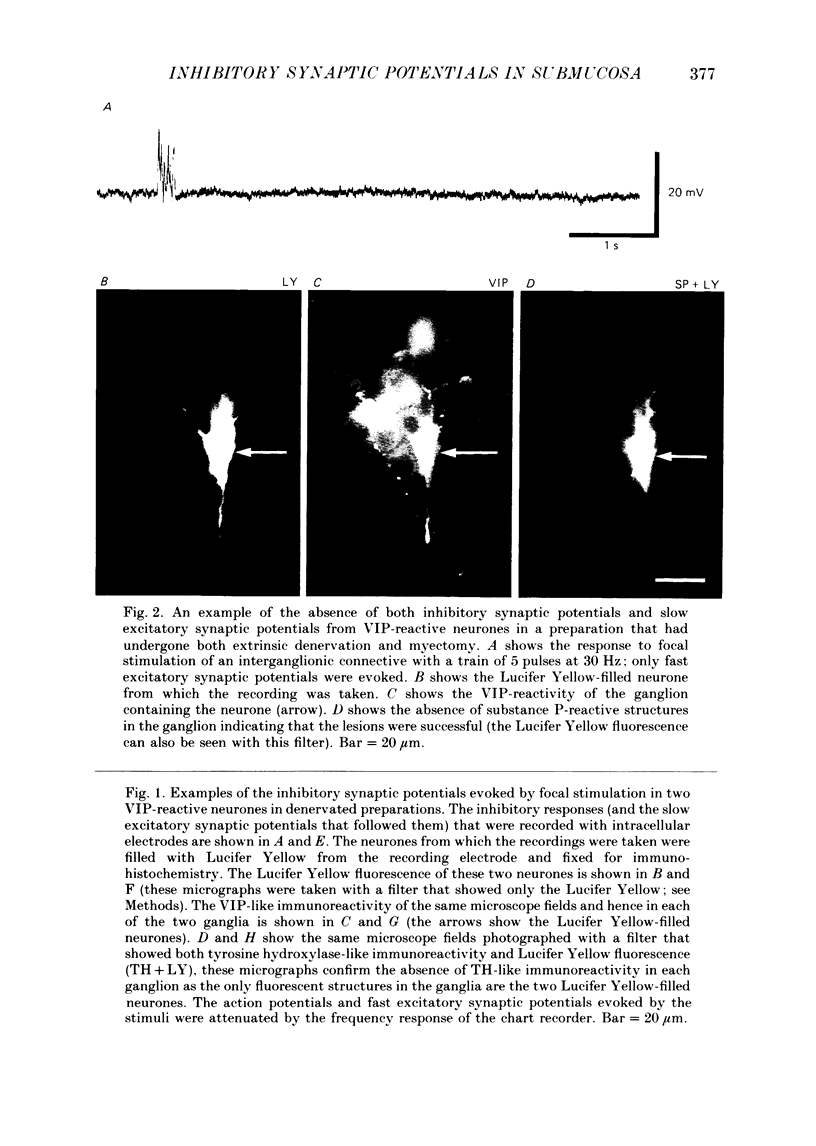
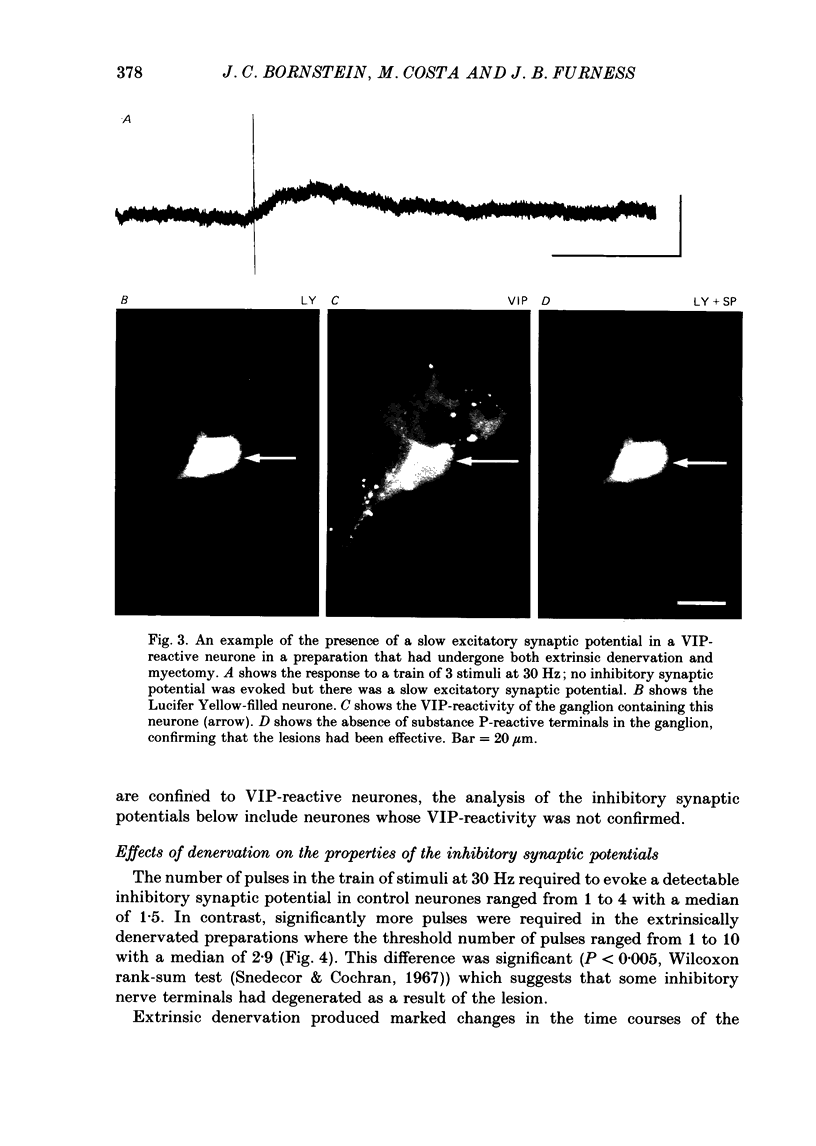
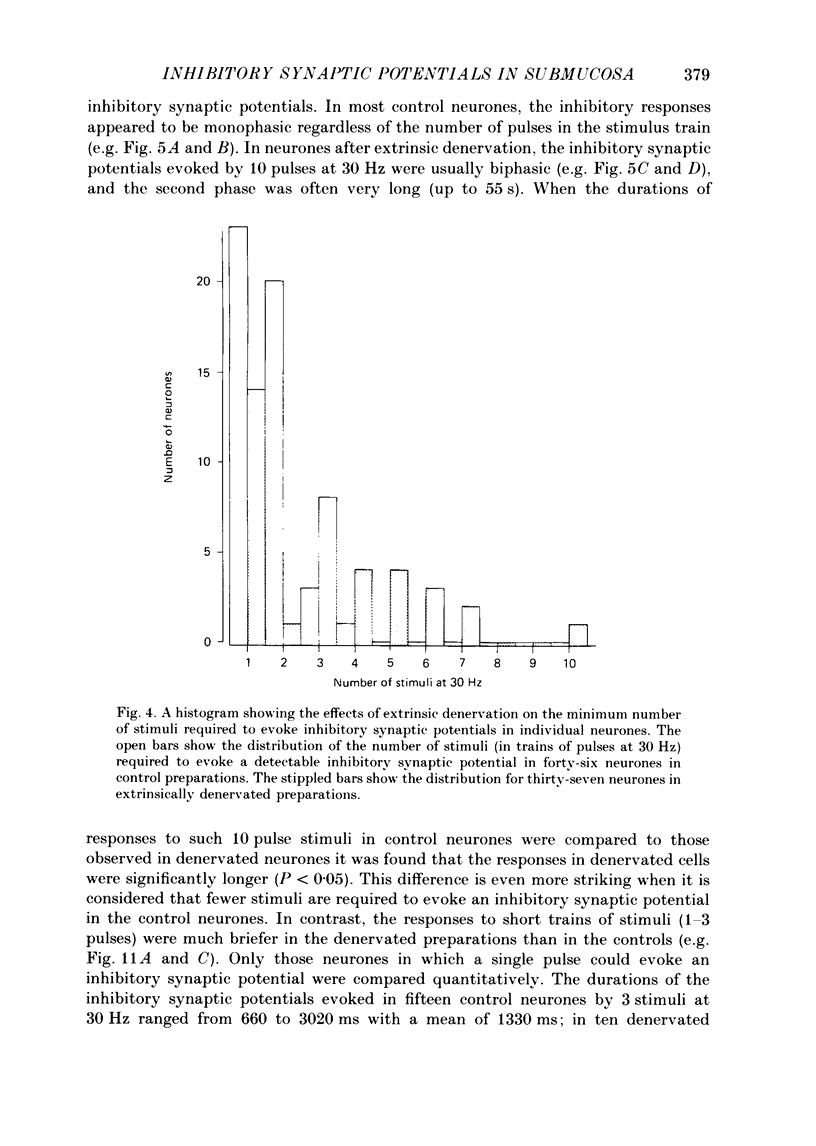
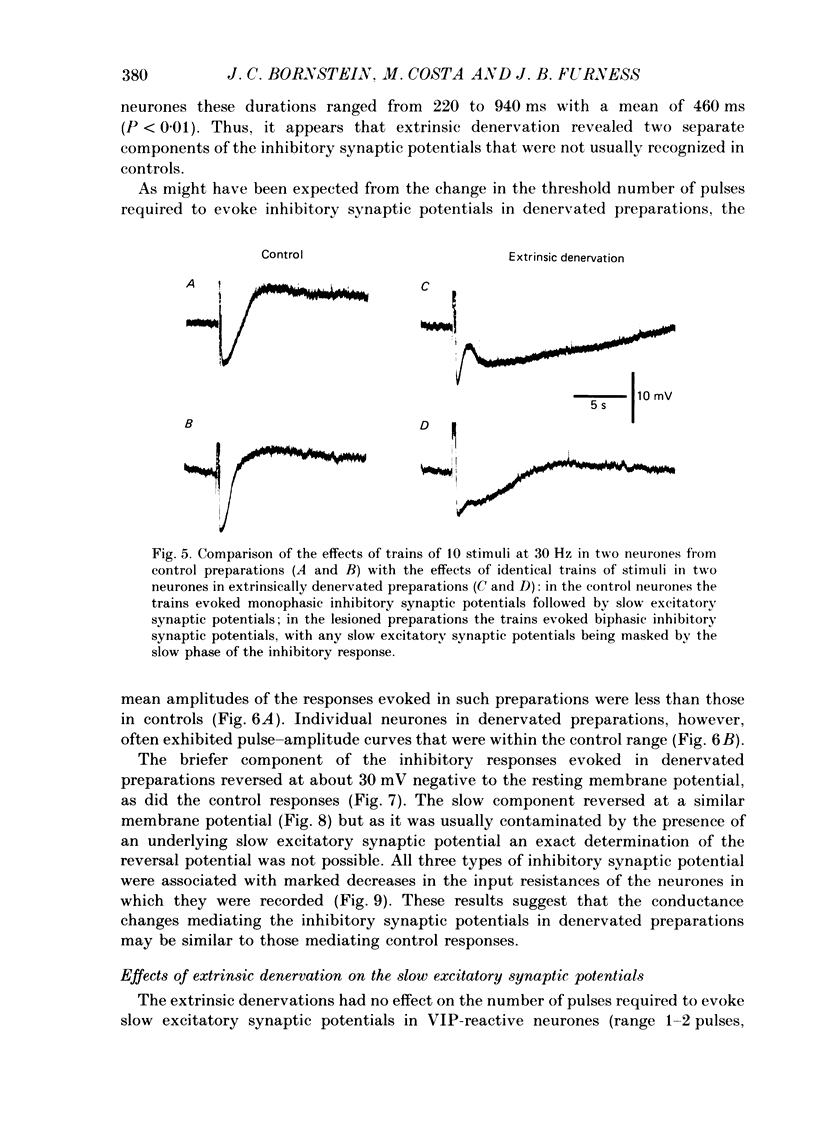
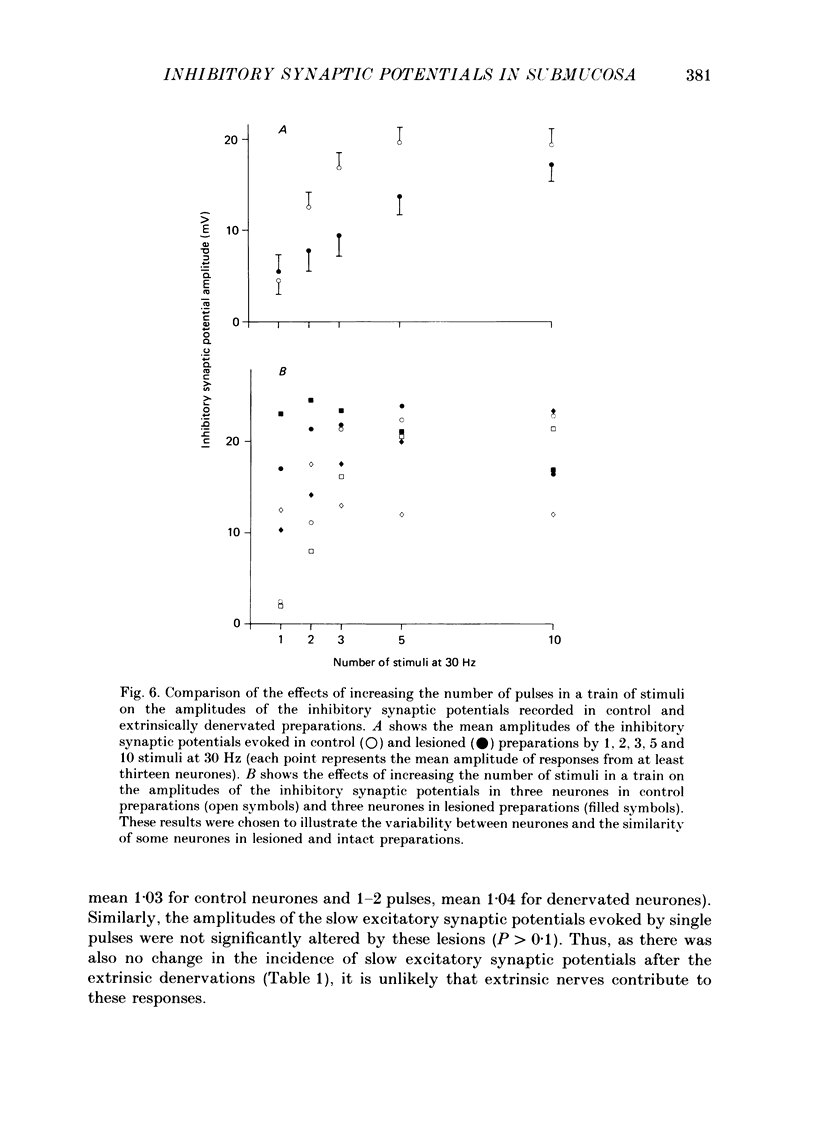
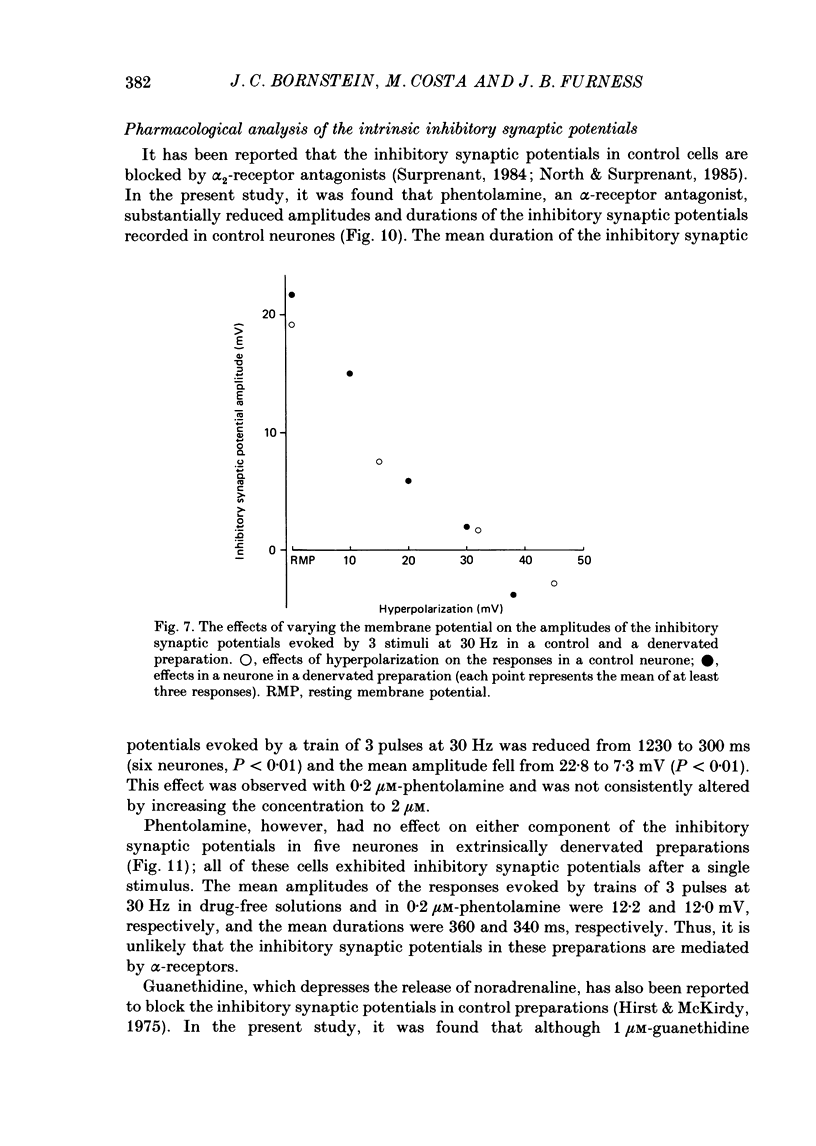
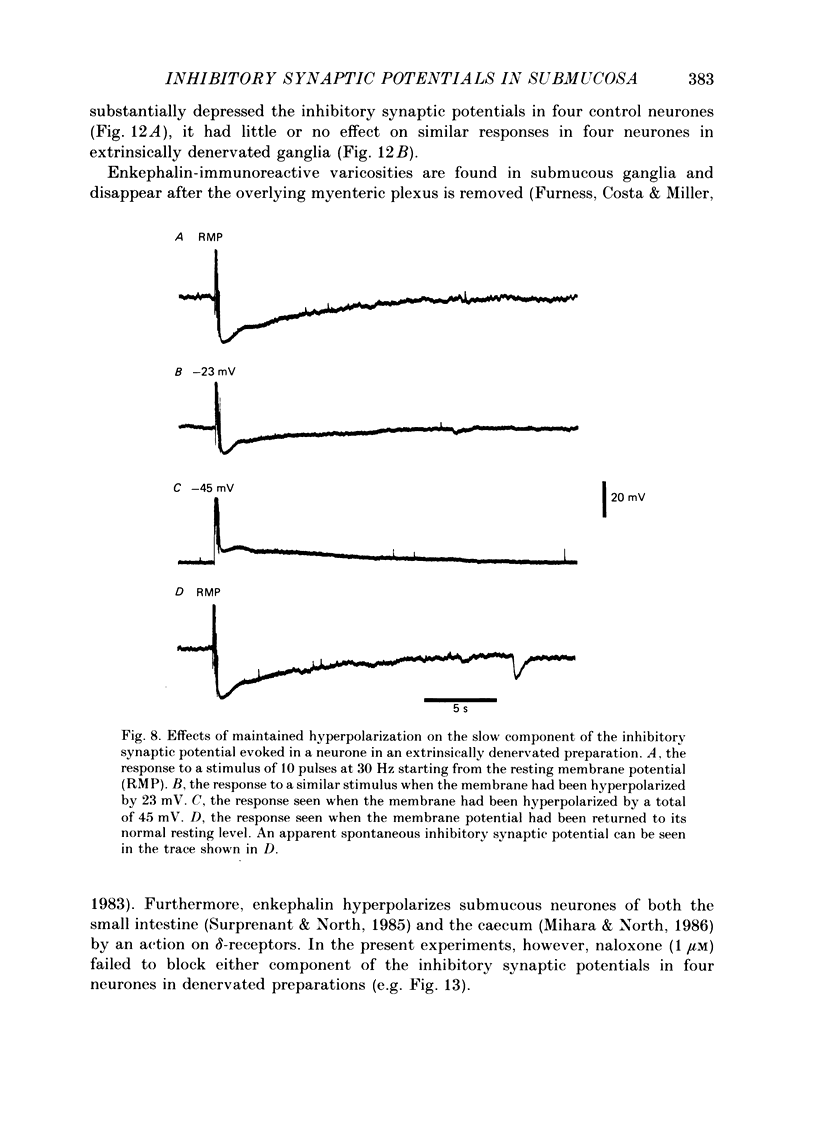
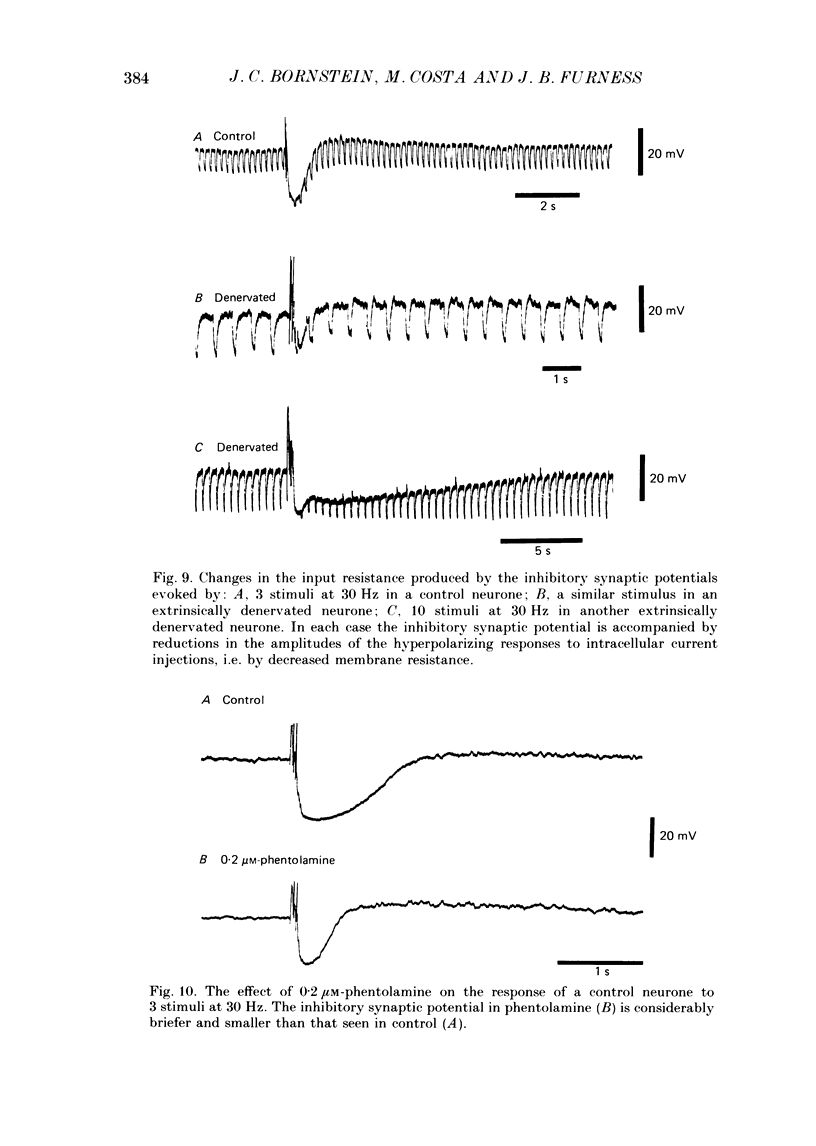



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein J. C., Costa M., Furness J. B., Lees G. M. Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurones of guinea-pig small intestine. J Physiol. 1984 Jun;351:313–325. doi: 10.1113/jphysiol.1984.sp015247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C., Costa M., Furness J. B. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol. 1986 Dec;381:465–482. doi: 10.1113/jphysiol.1986.sp016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C., Furness J. B., Costa M. Sources of excitatory synaptic inputs to neurochemically identified submucous neurons of guinea-pig small intestine. J Auton Nerv Syst. 1987 Jan;18(1):83–91. doi: 10.1016/0165-1838(87)90137-8. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Llewellyn-Smith I. J., Cuello A. C. Projections of substance P-containing neurons within the guinea-pig small intestine. Neuroscience. 1981;6(3):411–424. doi: 10.1016/0306-4522(81)90134-2. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984 Nov;13(3):911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):665–676. doi: 10.1016/0306-4522(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Galfre G., Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Freeman C. G. Absence of tyrosine hydroxylase activity and dopamine beta-hydroxylase immunoreactivity in intrinsic nerves of the guinea-pig ileum. Neuroscience. 1979;4(2):305–310. doi: 10.1016/0306-4522(79)90091-5. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Keast J. R. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res. 1984;237(2):329–336. doi: 10.1007/BF00217152. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Miller R. J. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):653–664. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Projections of intestinal neurons showing immunoreactivity for vasoactive intestinal polypeptide are consistent with these neurons being the enteric inhibitory neurons. Neurosci Lett. 1979 Dec;15(2-3):199–204. doi: 10.1016/0304-3940(79)96113-5. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe P. R., Provis J. C., Furness J. B., Costa M., Chalmers J. P. Residual catecholamines in extrinsically denervated guinea-pig ileum. Clin Exp Pharmacol Physiol. 1981 Jul;8(4):327–333. doi: 10.1111/j.1440-1681.1981.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J Physiol. 1975 Jul;249(2):269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast J. R., Furness J. B., Costa M. Effects of noradrenaline and somatostatin on basal and stimulated mucosal ion transport in the guinea-pig small intestine. Naunyn Schmiedebergs Arch Pharmacol. 1986 Aug;333(4):393–399. doi: 10.1007/BF00500015. [DOI] [PubMed] [Google Scholar]
- Keast J. R. Mucosal innervation and control of water and ion transport in the intestine. Rev Physiol Biochem Pharmacol. 1987;109:1–59. doi: 10.1007/BFb0031024. [DOI] [PubMed] [Google Scholar]
- Macrae I. M., Furness J. B., Costa M. Distribution of subgroups of noradrenaline neurons in the coeliac ganglion of the guinea-pig. Cell Tissue Res. 1986;244(1):173–180. doi: 10.1007/BF00218395. [DOI] [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986 Jun;88(2):315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., North R. A. mu-Opioid receptors and alpha 2-adrenoceptors coexist on myenteric but not on submucous neurones. Neuroscience. 1985 Oct;16(2):425–430. doi: 10.1016/0306-4522(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]