Abstract
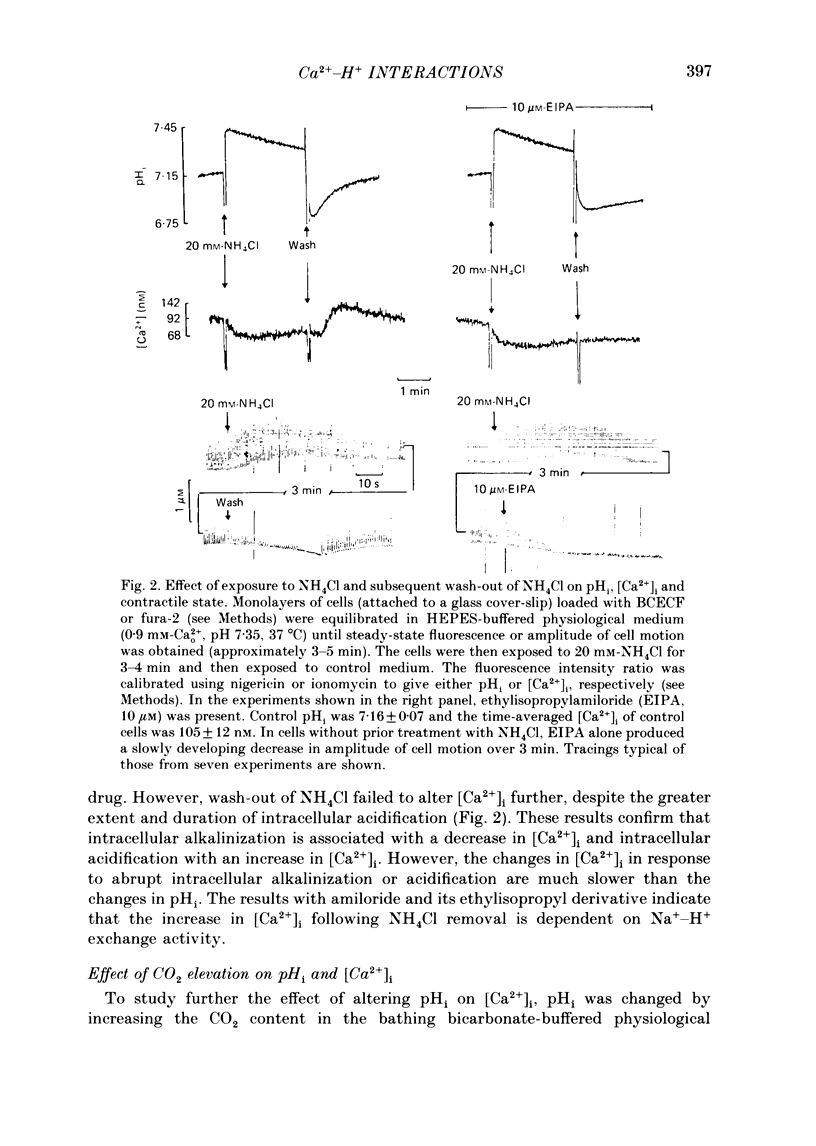
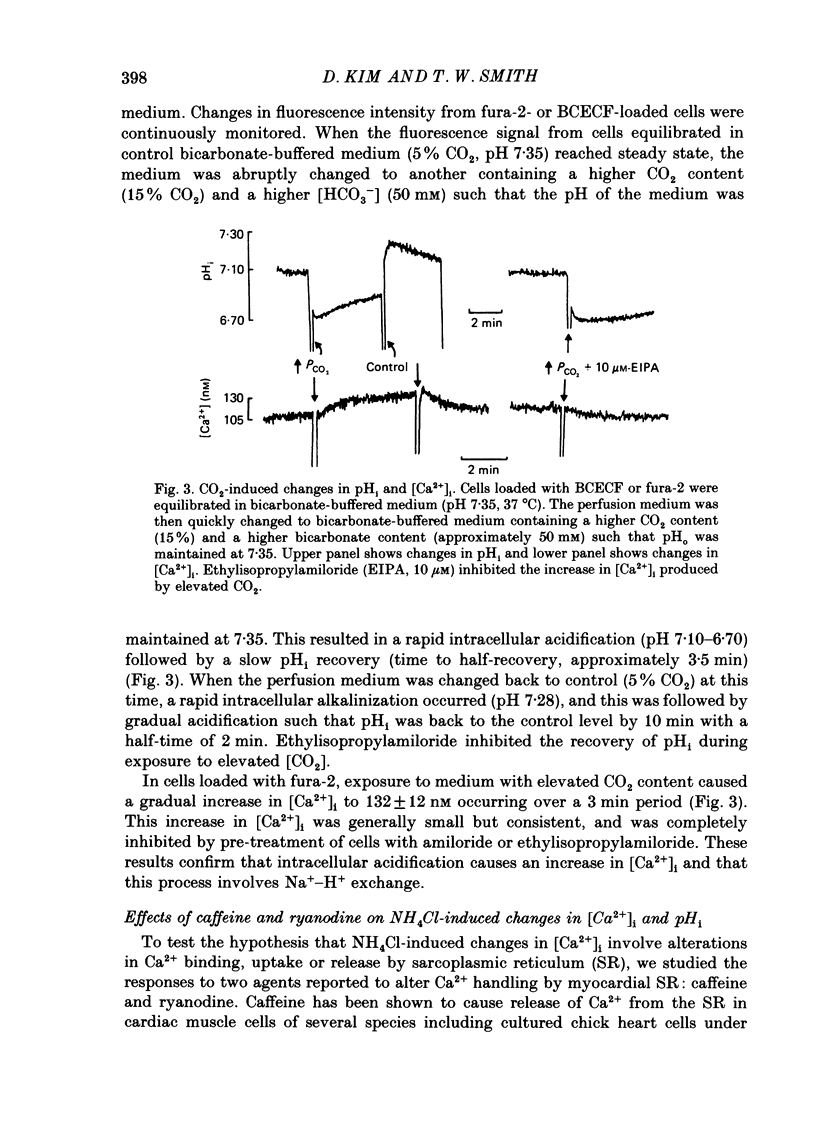
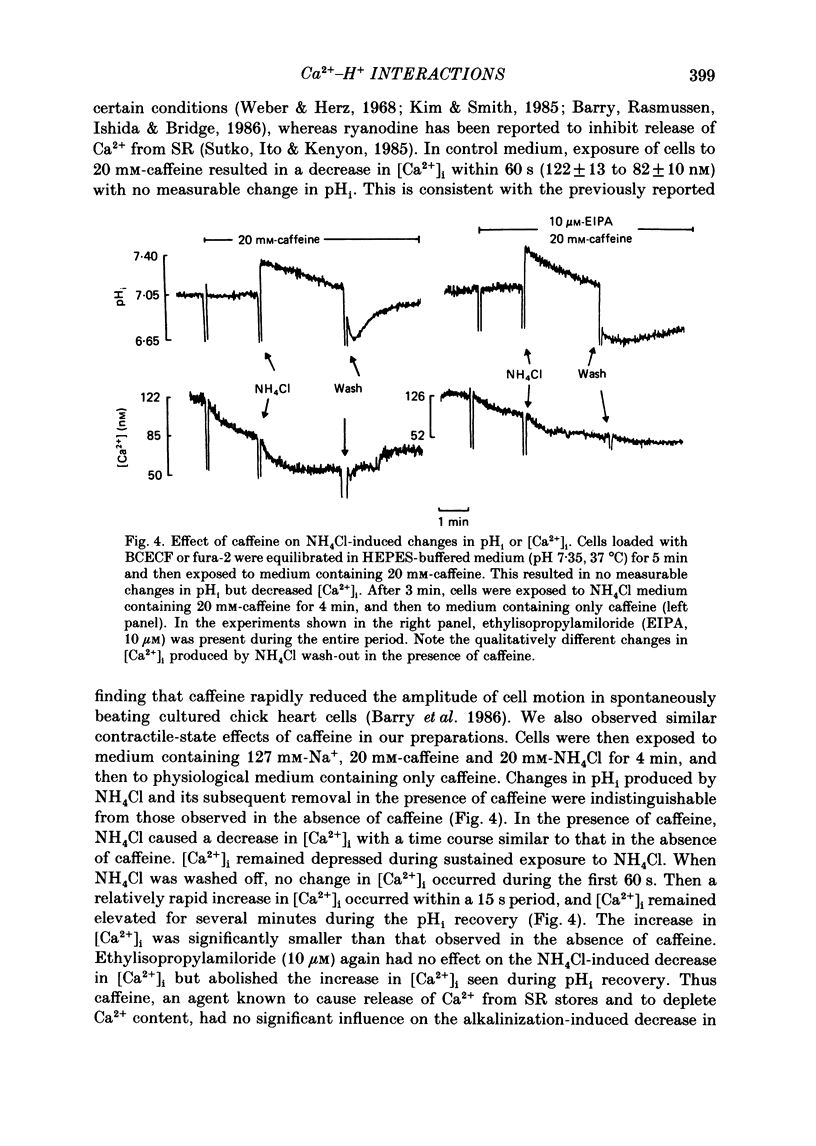
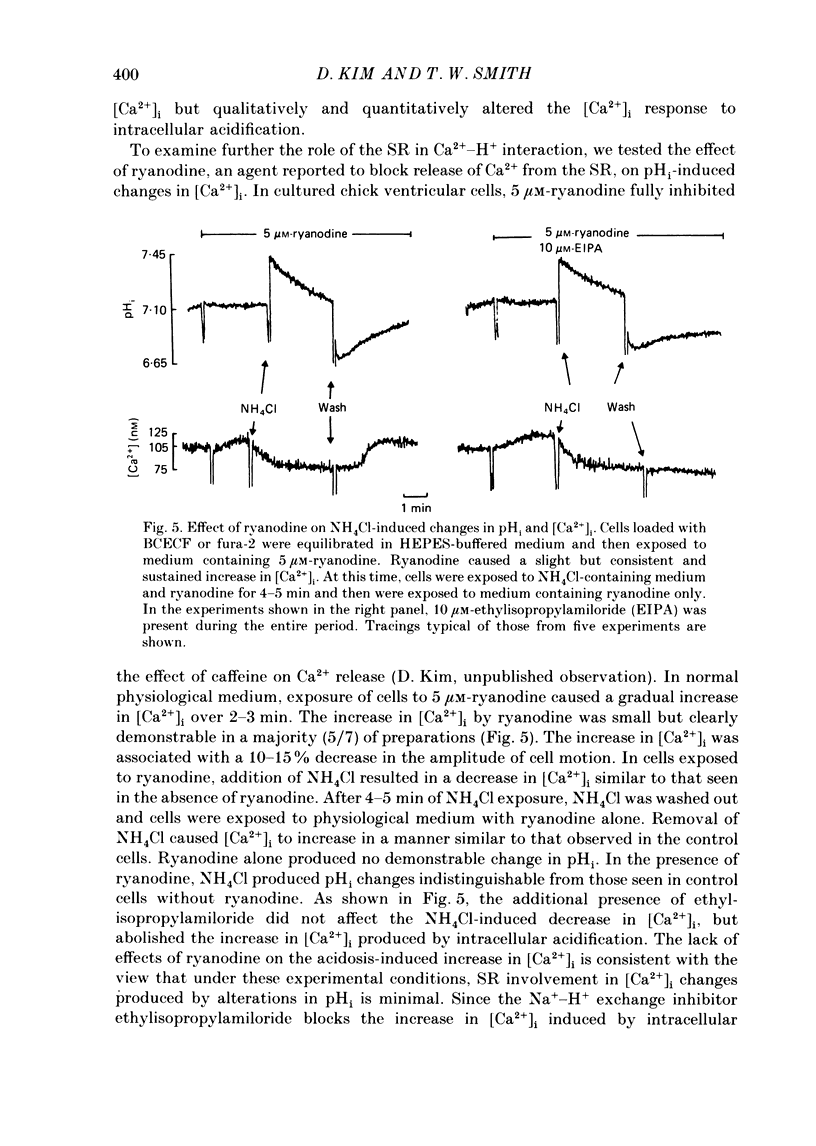
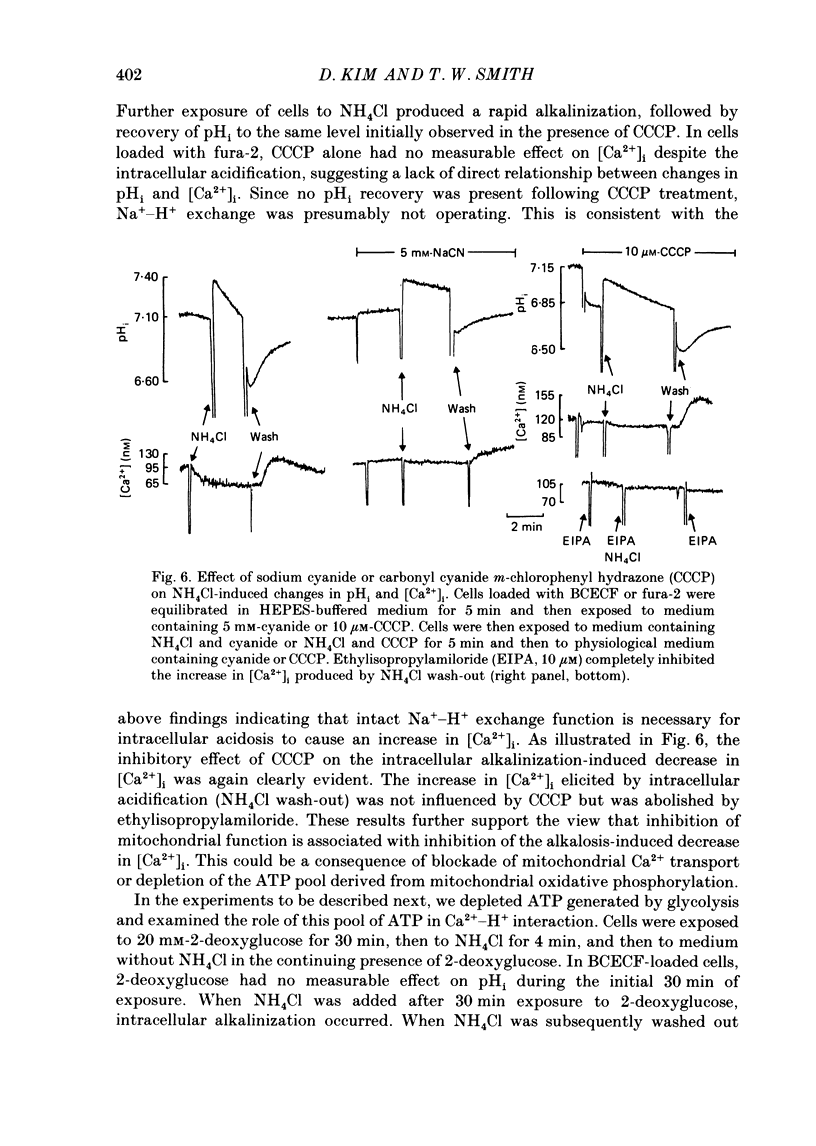
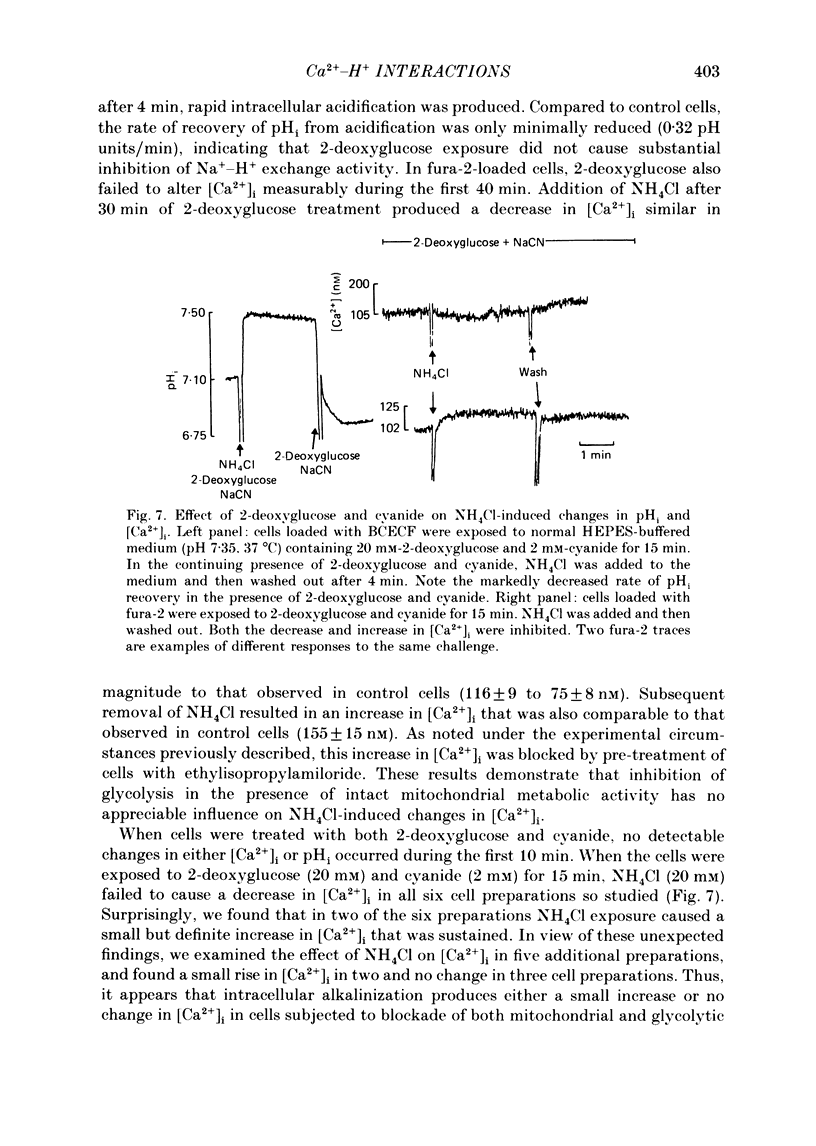
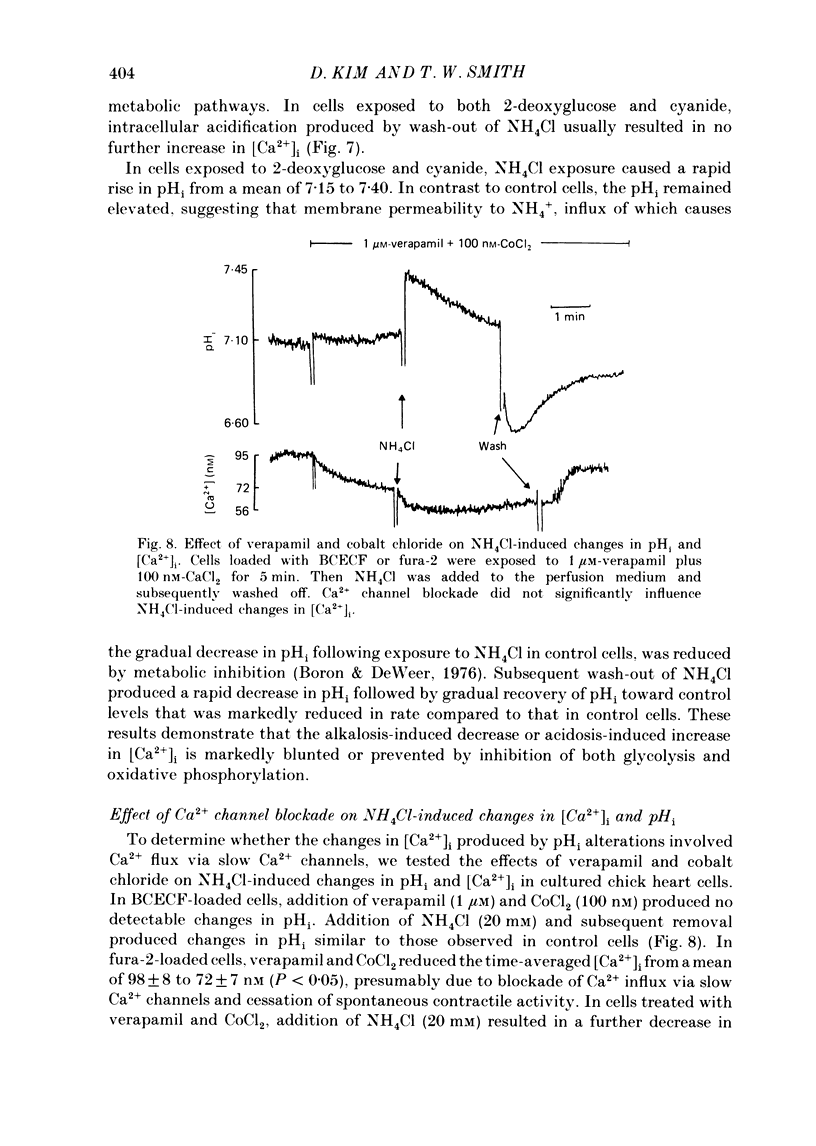
1. Cytosolic free Ca2+ concentration ([Ca2+]i) in cardiac muscle cells is influenced by many factors including intracellular pH. Intracellular alkalinization has been shown to reduce, whereas acidification has been shown to augment [Ca2+]i. We examined the cellular mechanisms underlying Ca2+-H+ interactions using cultured chick embryo ventricular cells. 2. Cells were loaded with fura-2 or BCECF (2,7-biscarboxyethyl-5(6)-carboxy-fluorescein) and changes in time-averaged [Ca2+]i or pHi were monitored continuously using a dual-wavelength spectrofluorometer. 3. Exposure of cells to 20 mM-NH4Cl (intracellular alkalinization) produced a rapid decrease in [Ca2+]i; subsequent wash-out of NH4Cl (intracellular acidification) resulted in an increase in [Ca2+]i to levels above control. Intracellular acidification produced by elevated CO2 content also resulted in an increase in [Ca2+]i. The Na+-H+ exchange inhibitor ethylisopropylamiloride (10 microM) inhibited completely the rise but not the fall in [Ca2+]i in response to manipulation of pHi with NH4Cl. 4. In the presence of caffeine (20 mM), NH4Cl produced a decrease in [Ca2+]i similar to that observed in the absence of caffeine, but subsequent removal of NH4Cl resulted in an increase in [Ca2+]i that was distinctly smaller than that observed in the absence of caffeine. Ryanodine (10 microM) had no significant influence on NH4Cl-induced changes in [Ca2+]i. 5. Following treatment with the mitochondrial inhibitors sodium cyanide (5 mM), CCCP (carbonyl cyanide m-chlorophenyl hydrazone, 10 microM) or rotenone (10 microM), the NH4Cl-induced decrease in [Ca2+]i was markedly diminished, but wash-out of NH4Cl resulted in increases in [Ca2+]i similar to those observed in control cells. 6. Inhibition of glycolysis with 20 mM-2-deoxyglucose did not significantly alter the changes in [Ca2+]i induced by NH4Cl addition or its wash-out, but 2-deoxyglucose plus cyanide abolished the decrease in [Ca2+]i produced by intracellular alkalinization and nearly completely blocked the increase in [Ca2+]i produced by acidification. 7. In all experiments, the increase in [Ca2+]i during wash-out of NH4Cl was inhibited by ethylisopropylamiloride.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Morris P. G., Pirolo J. S., Smith G. L. Metabolic consequences of increasing intracellular calcium and force production in perfused ferret hearts. J Physiol. 1986 Jul;376:121–141. doi: 10.1113/jphysiol.1986.sp016145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Honerjäger P. Influence of carbon dioxide on level of ionised calcium in squid axons. Nature. 1978 May 11;273(5658):160–161. doi: 10.1038/273160a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H., Rasmussen C. A., Jr, Ishida H., Bridge J. H. External Na-independent Ca extrusion in cultured ventricular cells. Magnitude and functional significance. J Gen Physiol. 1986 Sep;88(3):393–411. doi: 10.1085/jgp.88.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H., Smith T. W. Mechanisms of transmembrane calcium movement in cultured chick embryo ventricular cells. J Physiol. 1982 Apr;325:243–260. doi: 10.1113/jphysiol.1982.sp014148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. The homeostasis of calcium in heart cells. J Mol Cell Cardiol. 1985 Mar;17(3):203–212. doi: 10.1016/s0022-2828(85)80003-1. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Chapman R. A. Sodium/calcium exchange and intracellular calcium buffering in ferret myocardium: an ion-sensitive micro-electrode study. J Physiol. 1986 Apr;373:163–179. doi: 10.1113/jphysiol.1986.sp016040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnais J. M., Coraboeuf E., Sauviat M. P., Vassas J. M. Sensitivity to H, Li and Mg ions of the slow inward sodium current in frog atrial fibres. J Mol Cell Cardiol. 1975 Sep;7(9):627–642. doi: 10.1016/0022-2828(75)90140-6. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Fischmeister R., DeHaan R. L. Caffeine-induced current in embryonic heart cells: time course and voltage dependence. Am J Physiol. 1983 Sep;245(3):H528–H532. doi: 10.1152/ajpheart.1983.245.3.H528. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. The role of the Na+/H+ exchange system in the regulation of the internal pH in cultured cardiac cells. Eur J Biochem. 1985 May 15;149(1):1–4. doi: 10.1111/j.1432-1033.1985.tb08884.x. [DOI] [PubMed] [Google Scholar]
- Fry C. H., Poole-Wilson P. A. Effects of acid-base changes on excitation--contraction coupling in guinea-pig and rabbit cardiac ventricular muscle. J Physiol. 1981;313:141–160. doi: 10.1113/jphysiol.1981.sp013655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hasin Y., Barry W. H. Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol. 1984 Aug;247(2 Pt 2):H322–H329. doi: 10.1152/ajpheart.1984.247.2.H322. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Hecht H. H. Editorial: the early "pump" failure of the ischemic heart. Am J Med. 1969 Oct;47(4):497–502. doi: 10.1016/0002-9343(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Kim D., Cragoe E. J., Jr, Smith T. W. Relations among sodium pump inhibition, Na-Ca and Na-H exchange activities, and Ca-H interaction in cultured chick heart cells. Circ Res. 1987 Feb;60(2):185–193. doi: 10.1161/01.res.60.2.185. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Altered Ca fluxes and contractile state during pH changes in cultured heart cells. Am J Physiol. 1987 Jul;253(1 Pt 1):C137–C146. doi: 10.1152/ajpcell.1987.253.1.C137. [DOI] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Effects of thyroid hormone on calcium handling in cultured chick ventricular cells. J Physiol. 1985 Jul;364:131–149. doi: 10.1113/jphysiol.1985.sp015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Haap K., Figulla H. R. Influence of low extracellular pH upon the Ca inward current and isometric contractile force in mammalian ventricular myocardium. Pflugers Arch. 1976 Oct 15;366(1):31–38. doi: 10.1007/BF02486557. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Relationship between myocardial contractility and the effects of digitalis on ionic exchange. Fed Proc. 1977 Aug;36(9):2231–2234. [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Carbon dioxide or bicarbonate ions release Ca2+ from internal stores in crustacean myofibrillar bundles. J Membr Biol. 1981;61(2):115–125. doi: 10.1007/BF02007638. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Bersohn M. M., Nishimoto A. Y. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ Res. 1982 Feb;50(2):287–293. doi: 10.1161/01.res.50.2.287. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells. pHi regulation. J Gen Physiol. 1985 Jan;85(1):43–64. doi: 10.1085/jgp.85.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi L., Bucx J. J., ter Keurs H. E. Effects of acidosis on force-sarcomere length and force-velocity relations of rat cardiac muscle. Cardiovasc Res. 1986 Feb;20(2):117–123. doi: 10.1093/cvr/20.2.117. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sperelakis N. Blockade of myocardial slow inward current at low pH. Am J Physiol. 1977 Sep;233(3):C99–103. doi: 10.1152/ajpcell.1977.233.3.C99. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


