Abstract
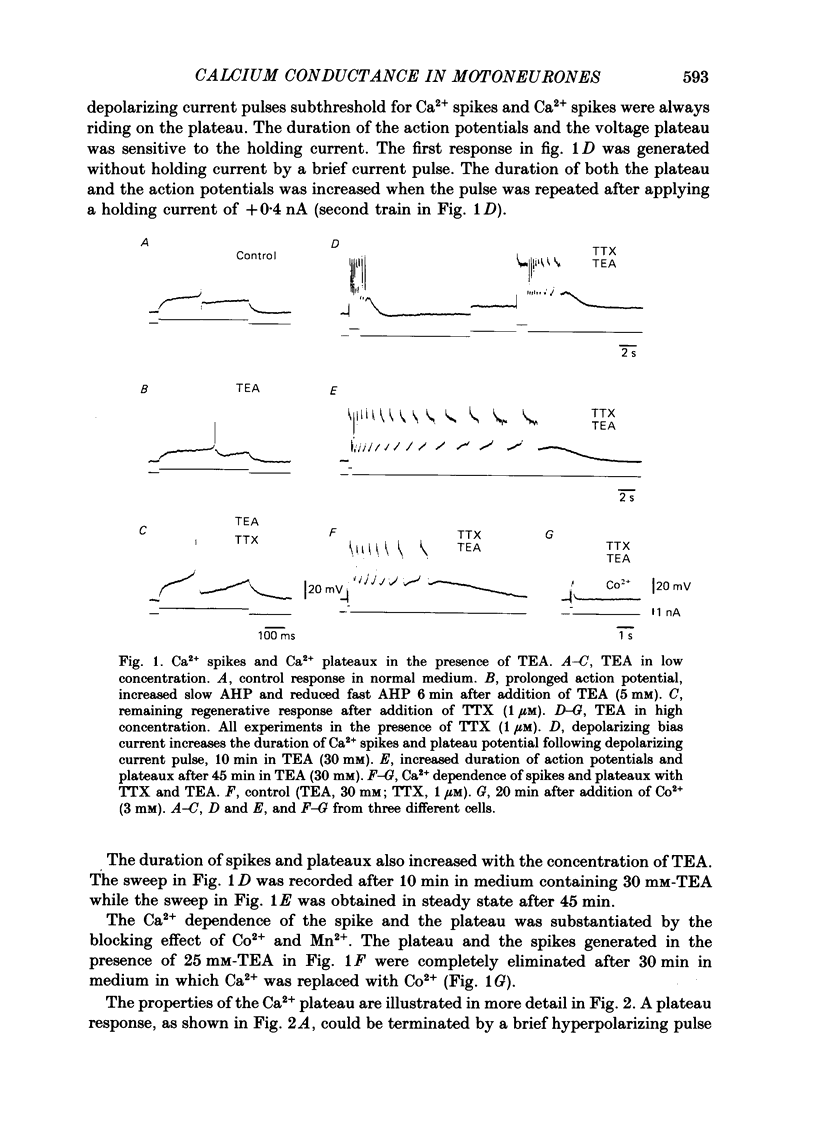
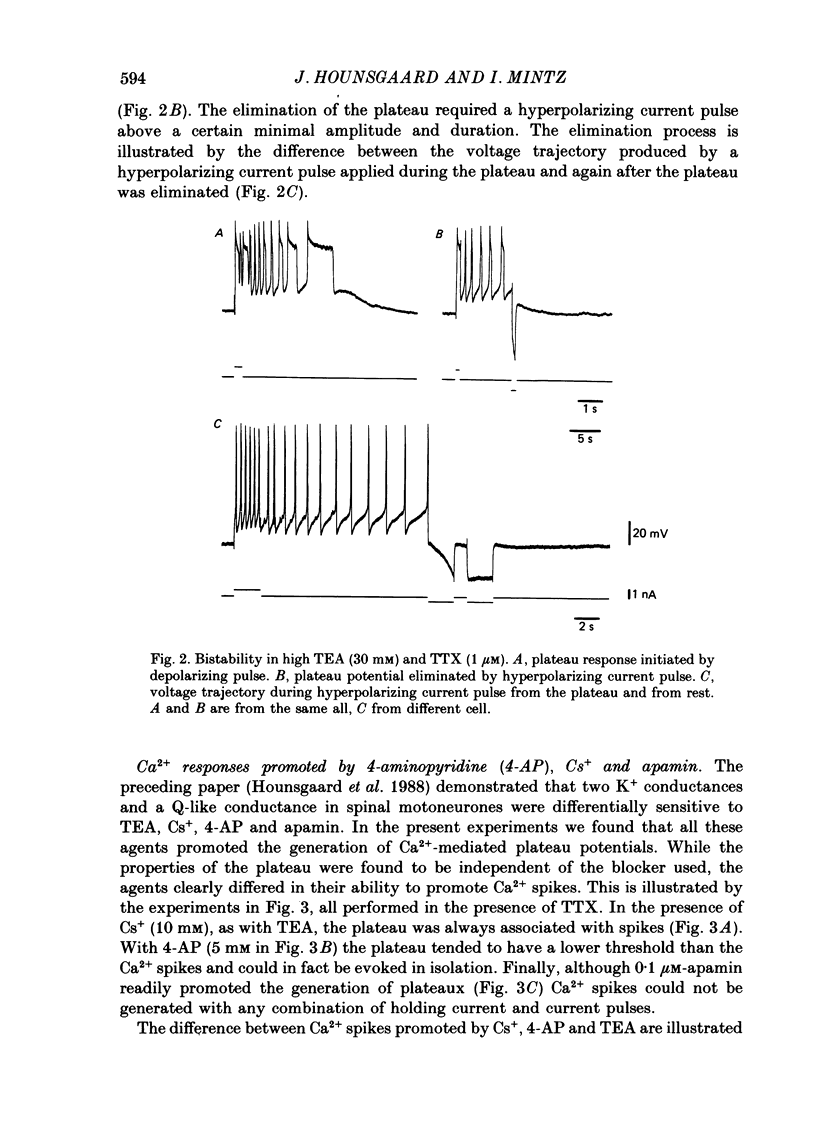
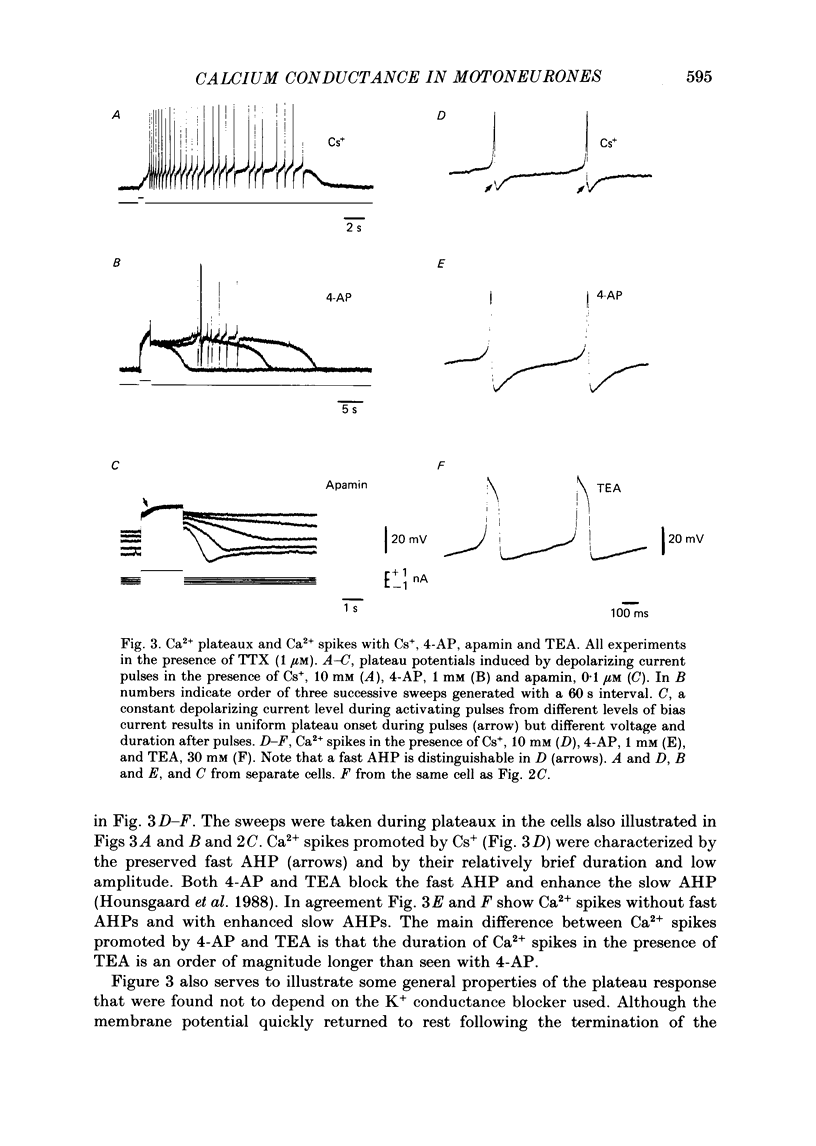
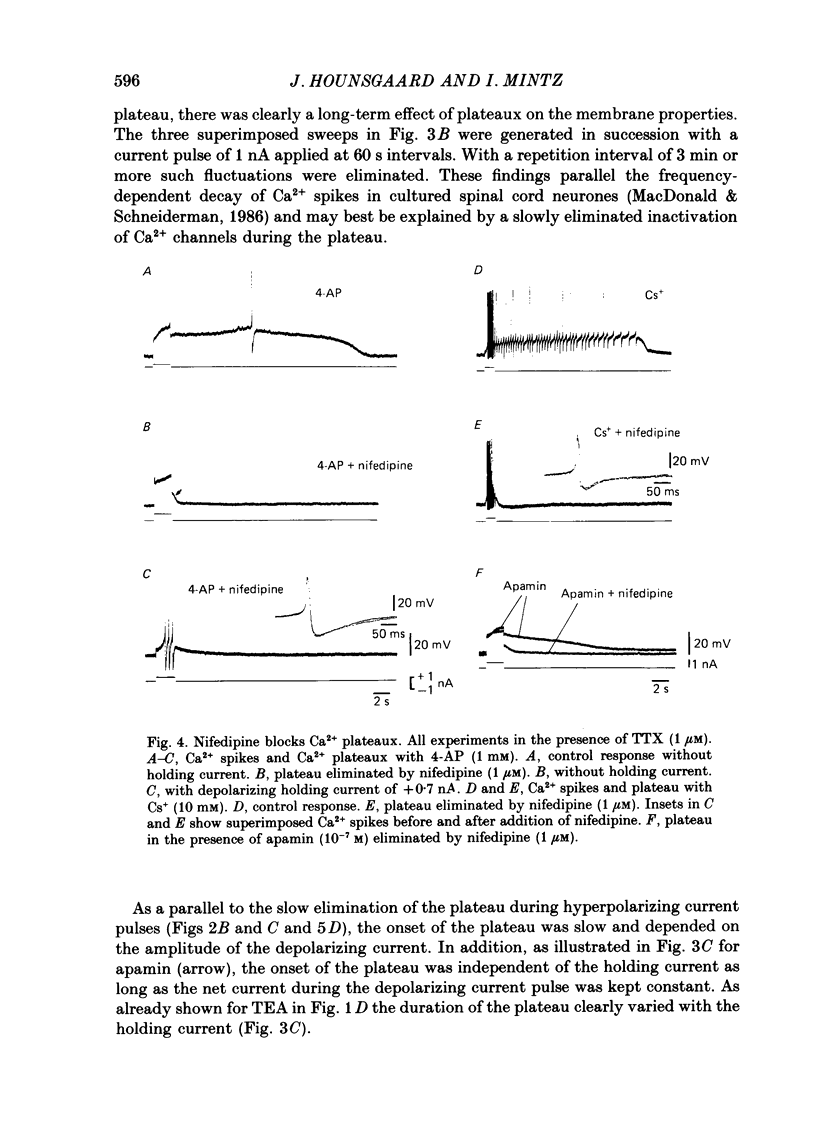
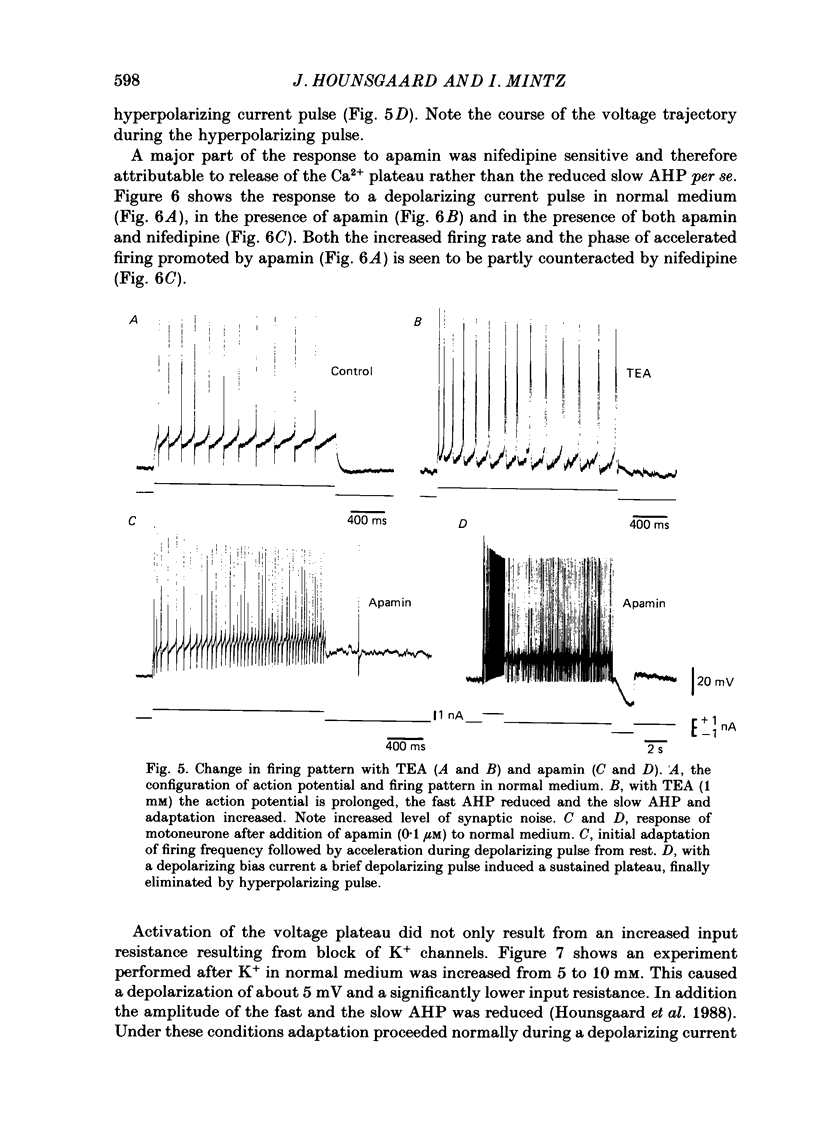
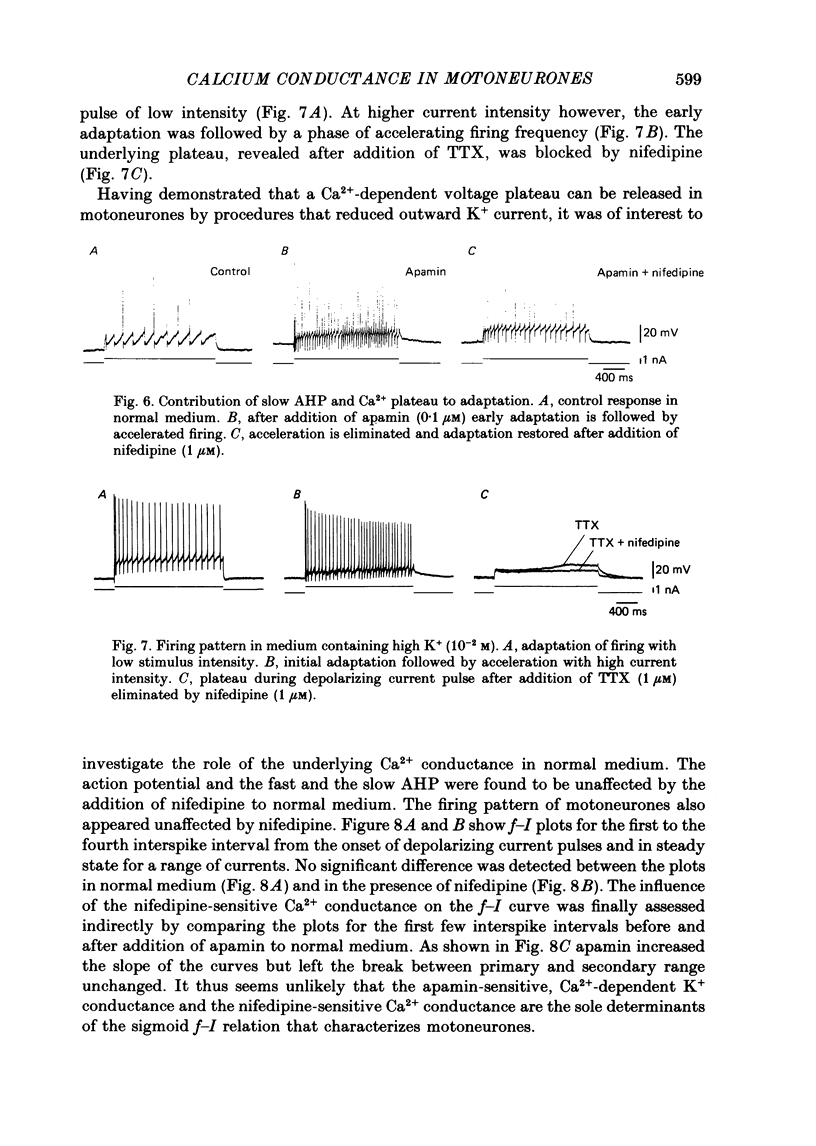
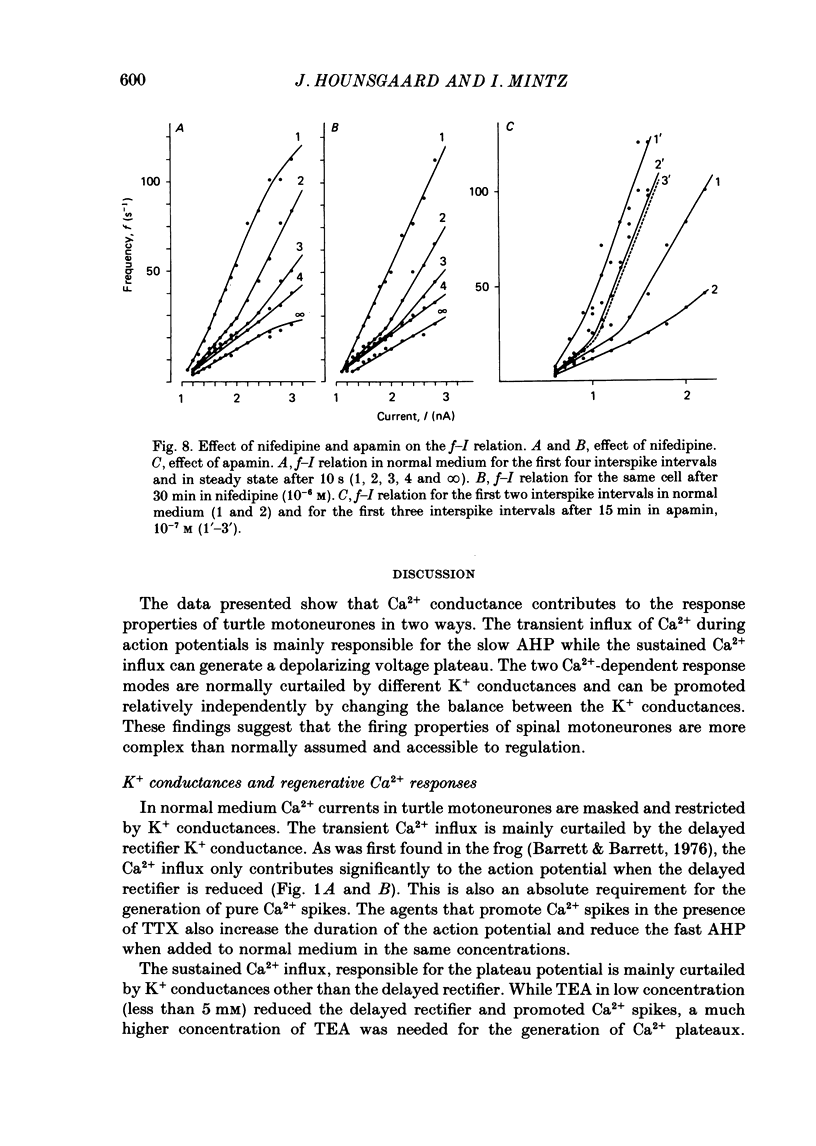
1. The contribution of Ca2+ conductance to the firing properties of motoneurones was investigated in transverse slices of the turtle spinal cord. 2. In the presence of tetrodotoxin (TTX), tetraethylamonium (TEA) in low extracellular concentration (less than 5 mM) promoted Ca2+ spikes. In higher concentrations of TEA, a suprathreshold depolarizing current pulse was followed by an after-discharge of Ca2+ spikes riding on a Ca2+ plateau potential. 3. The Ca2+-dependent plateau was also promoted by Cs+, 4-aminopyridine (4-AP) and apamin. However, Ca2+ spikes during plateaux were an order of magnitude faster when promoted by Cs+ or 4-AP rather than TEA, and apamin did not promote Ca2+ spikes at all. 4. Ca2+ plateaux but not Ca2+ spikes were blocked by nifedipine. 5. In normal medium all effects of the transient Ca2+ influx during action potentials were attributable to its influence on the slow after-hyperpolarization. The nifedipine-sensitive, sustained Ca2+ influx was expressed exclusively as plateau potentials and only under conditions of reduced K+ current. 6. It is concluded that the transient and the sustained Ca2+ fluxes in spinal motoneurones are curtailed by different K+ conductances. The two Ca2+ responses are suggested as being mediated by two different types of Ca2+ channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldissera F., Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course. First interval firing. Acta Physiol Scand. 1974 Aug;91(4):528–544. doi: 10.1111/j.1748-1716.1974.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H., Schwindt P. C. Steps in production of motoneuron spikes during rhythmic firing. J Neurophysiol. 1972 May;35(3):297–310. doi: 10.1152/jn.1972.35.3.297. [DOI] [PubMed] [Google Scholar]
- Harada Y., Takahashi T. The calcium component of the action potential in spinal motoneurones of the rat. J Physiol. 1983 Feb;335:89–100. doi: 10.1113/jphysiol.1983.sp014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57(2):422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O., Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988 Apr;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Schneiderman J. H. Frequency-dependent decay of calcium spikes in cultured spinal cord neurons. Neuroscience. 1986 Dec;19(4):1335–1347. doi: 10.1016/0306-4522(86)90147-8. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D., Tsien R. W. Different types of calcium channels. J Exp Biol. 1986 Sep;124:177–190. doi: 10.1242/jeb.124.1.177. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980 Jun;43(6):1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W., Crill W. E. Epileptogenic action of tungstic acid gel on cat lumbar motoneurons. Brain Res. 1984 Jan 16;291(1):140–144. doi: 10.1016/0006-8993(84)90660-7. [DOI] [PubMed] [Google Scholar]
- Schwindt P., Crill W. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980 May;43(5):1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Walton K., Fulton B. P. Ionic mechanisms underlying the firing properties of rat neonatal motoneurons studied in vitro. Neuroscience. 1986 Nov;19(3):669–683. doi: 10.1016/0306-4522(86)90291-5. [DOI] [PubMed] [Google Scholar]
- Zhang L., Krnjević K. Apamin depresses selectively the after-hyperpolarization of cat spinal motoneurons. Neurosci Lett. 1987 Feb 10;74(1):58–62. doi: 10.1016/0304-3940(87)90051-6. [DOI] [PubMed] [Google Scholar]


