Abstract
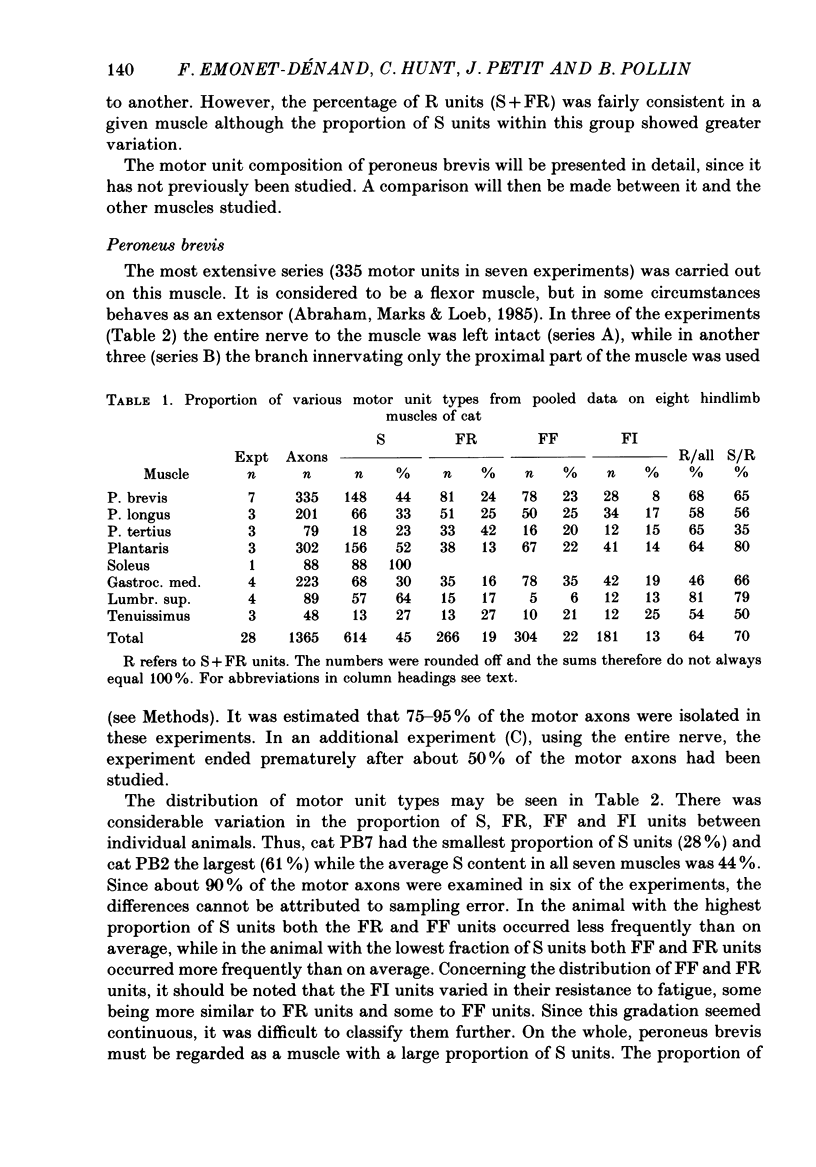
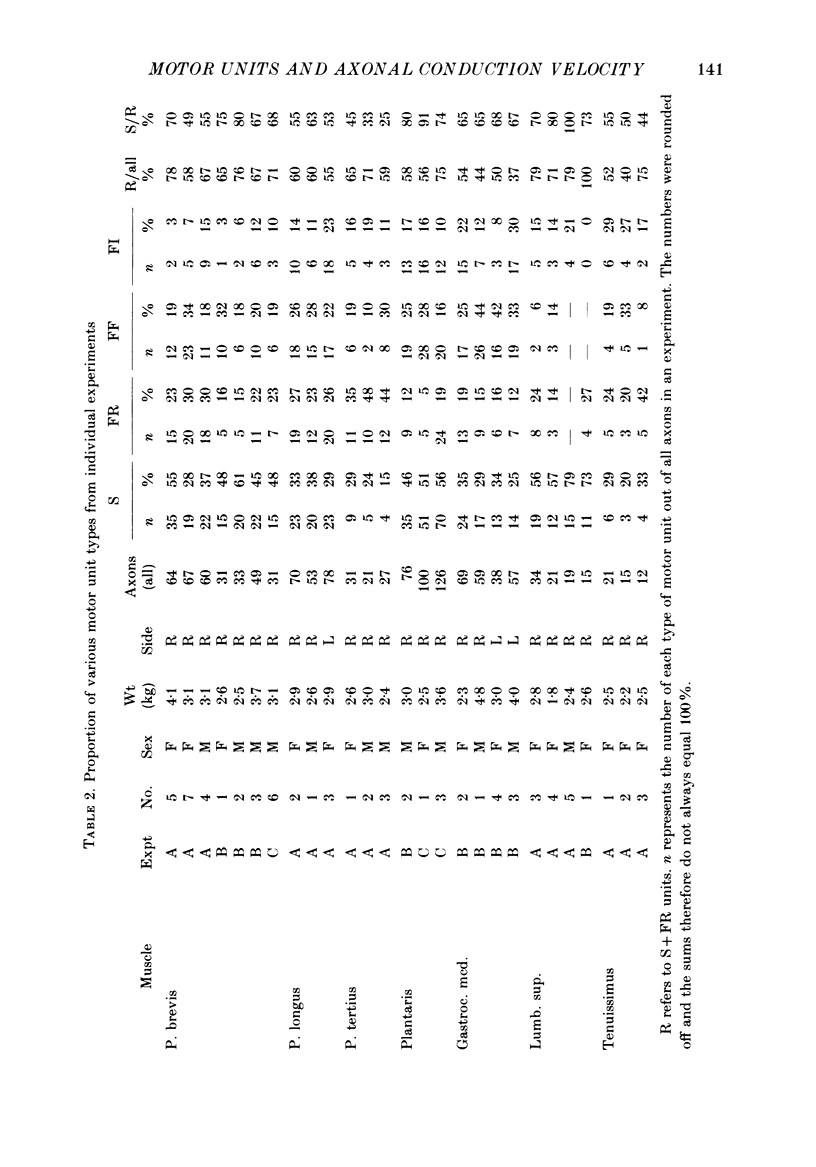
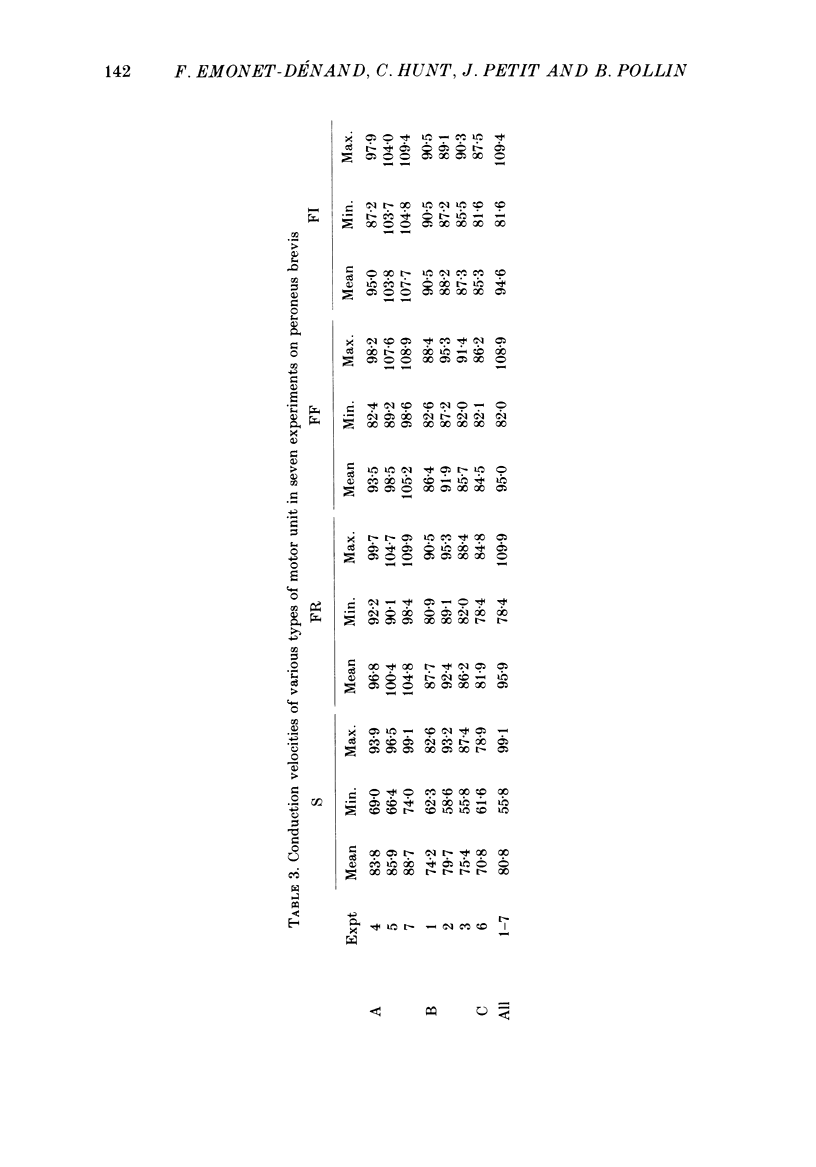
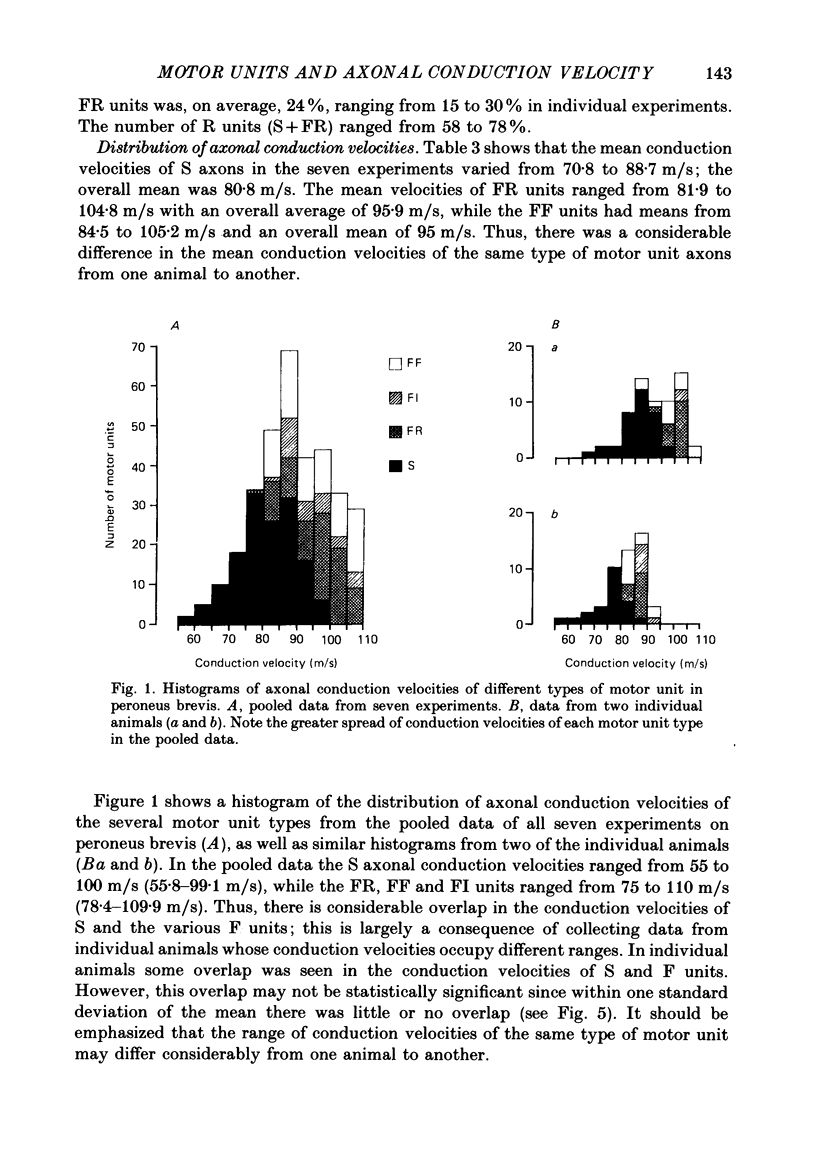
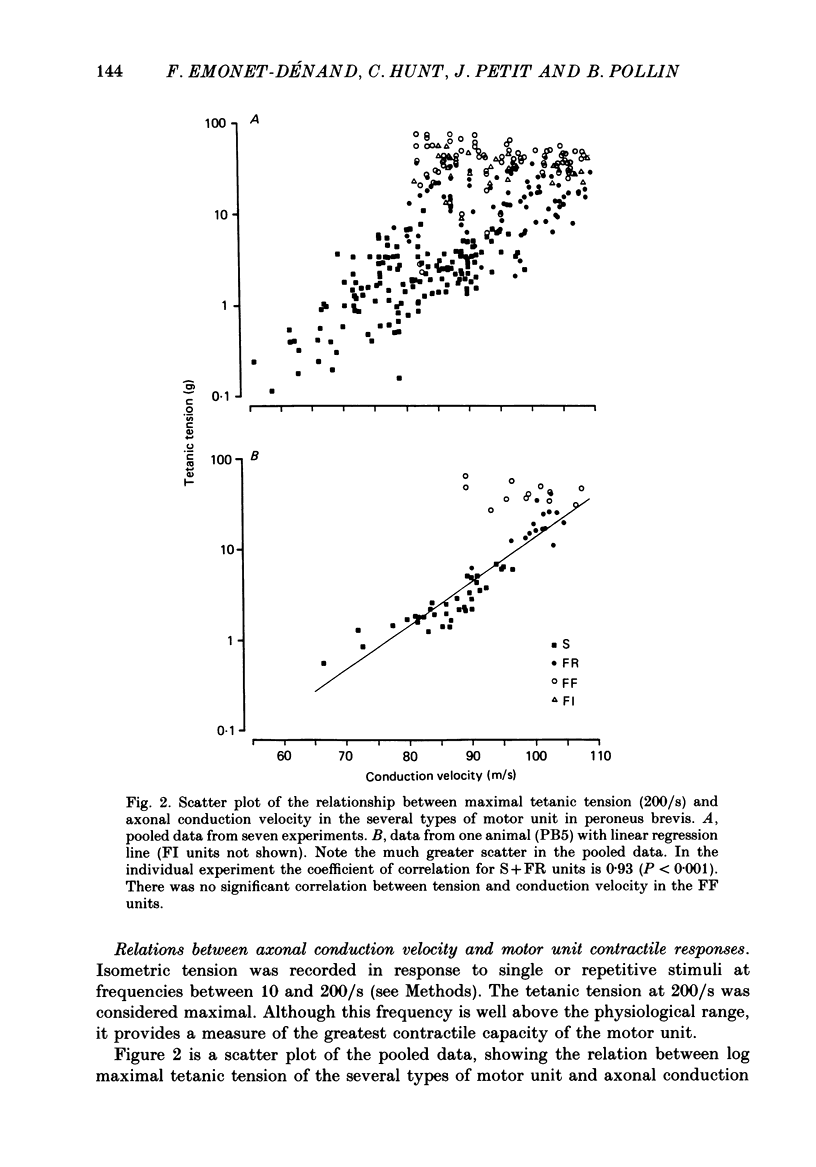
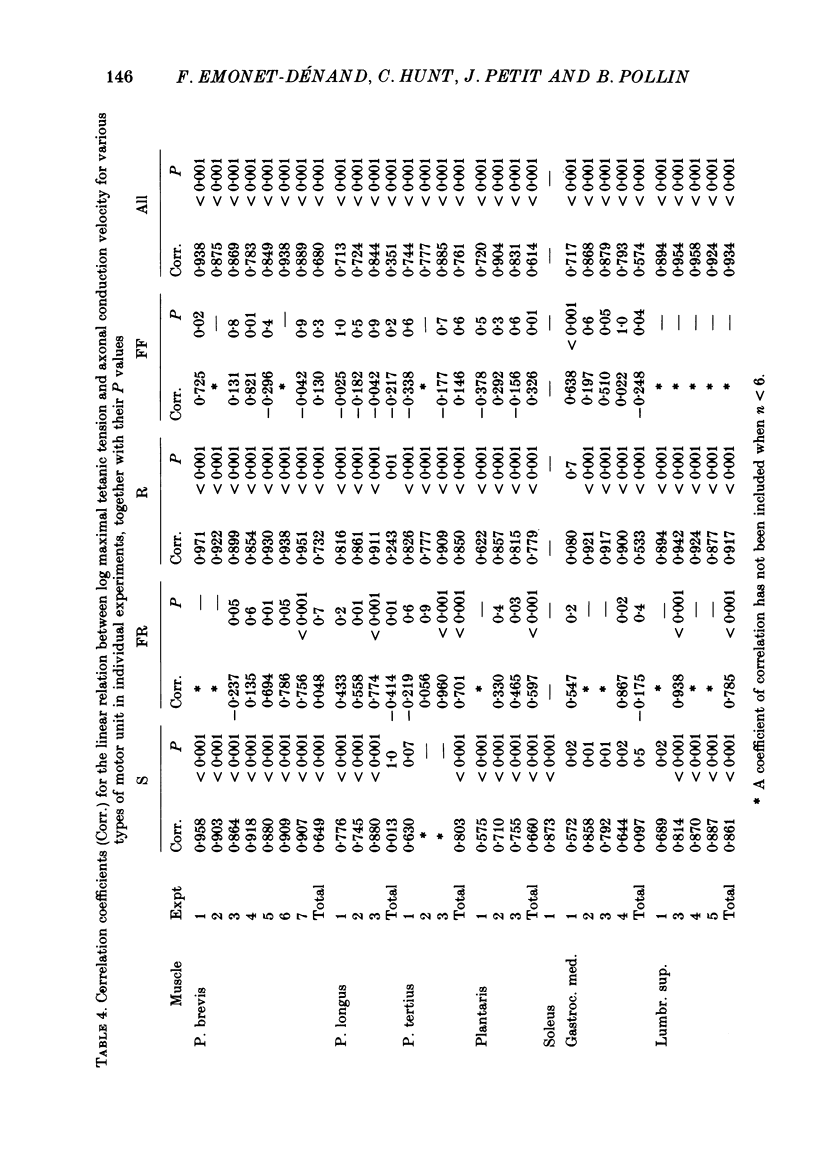
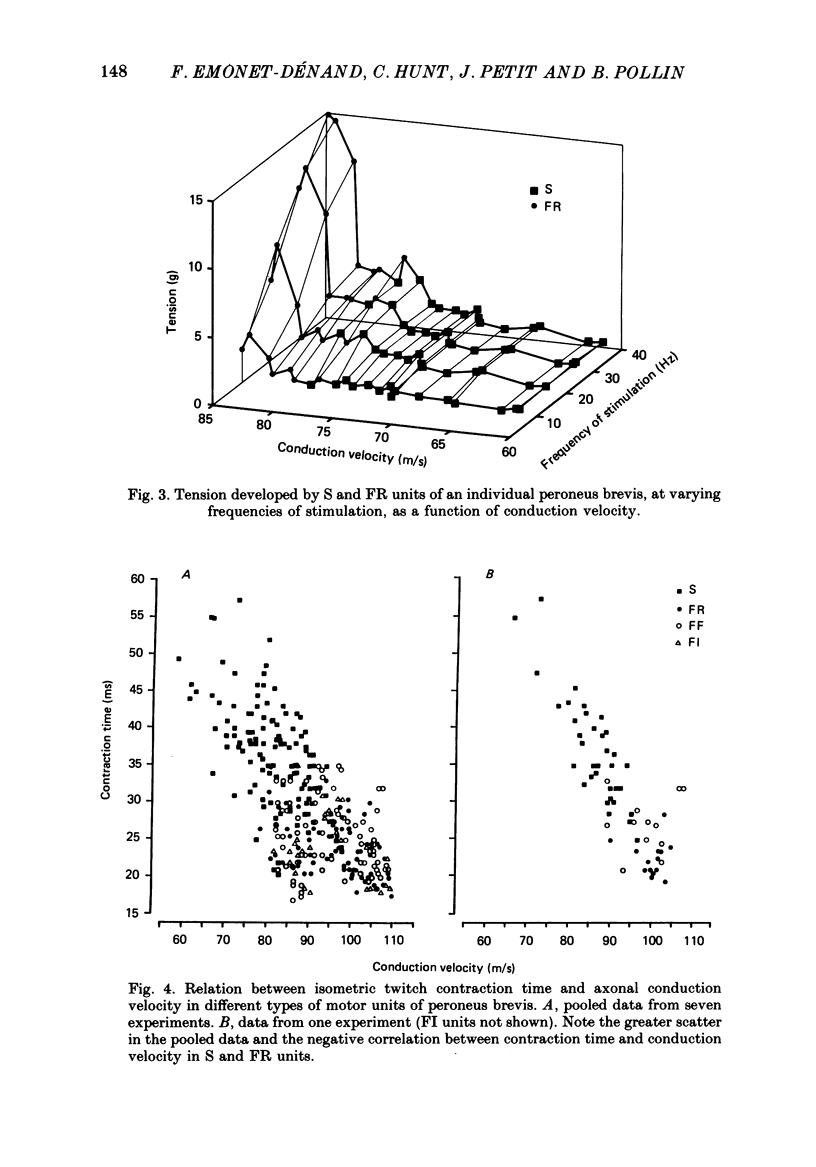
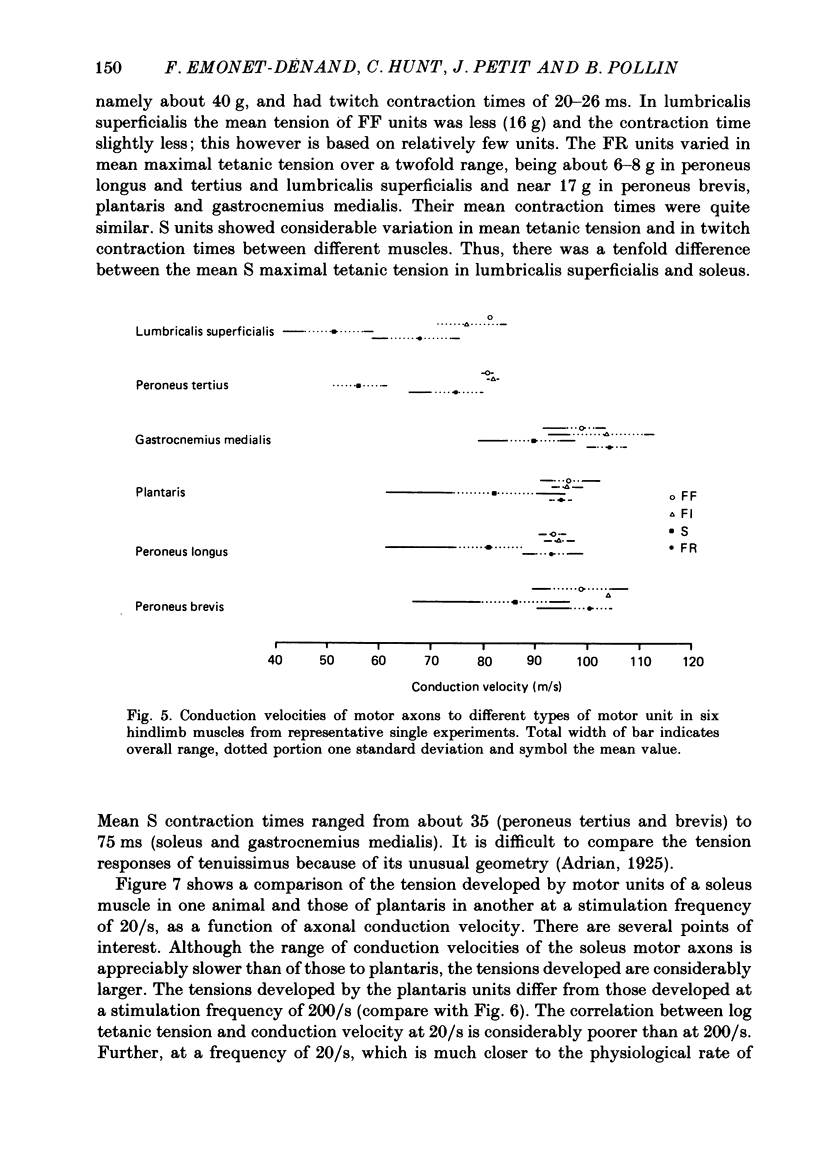
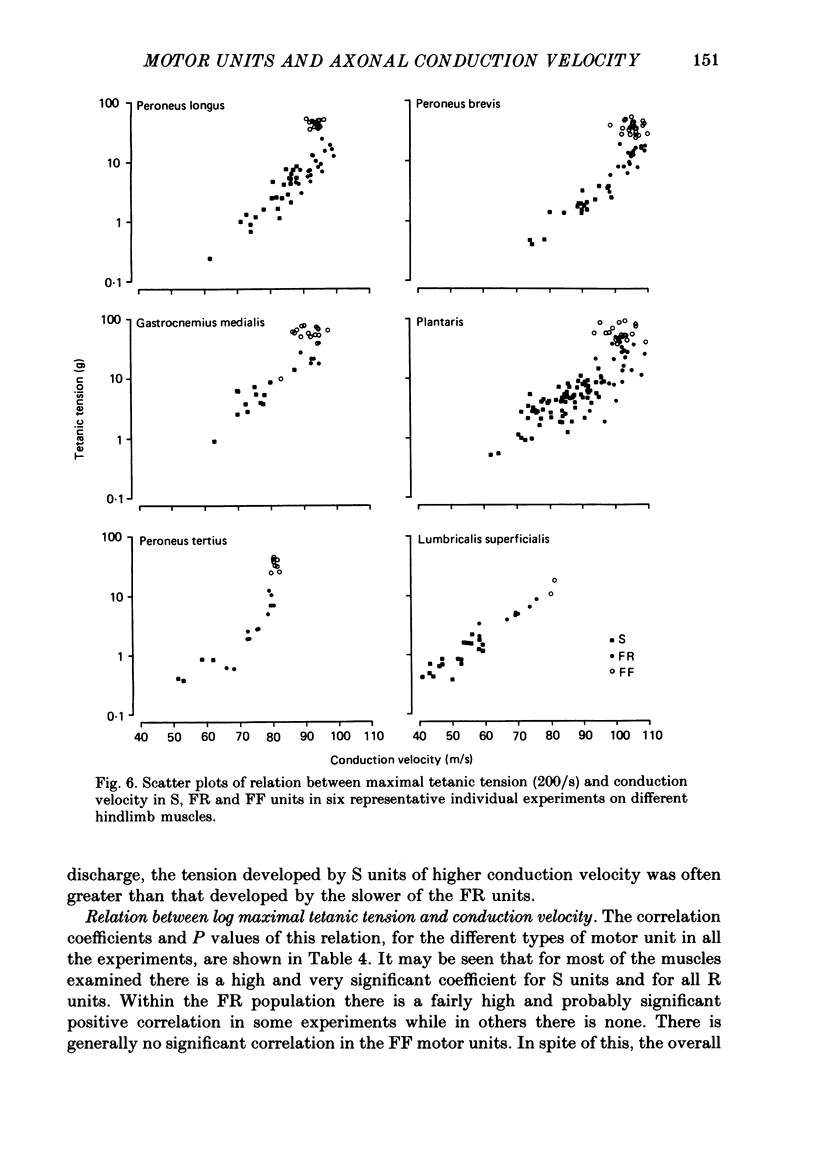
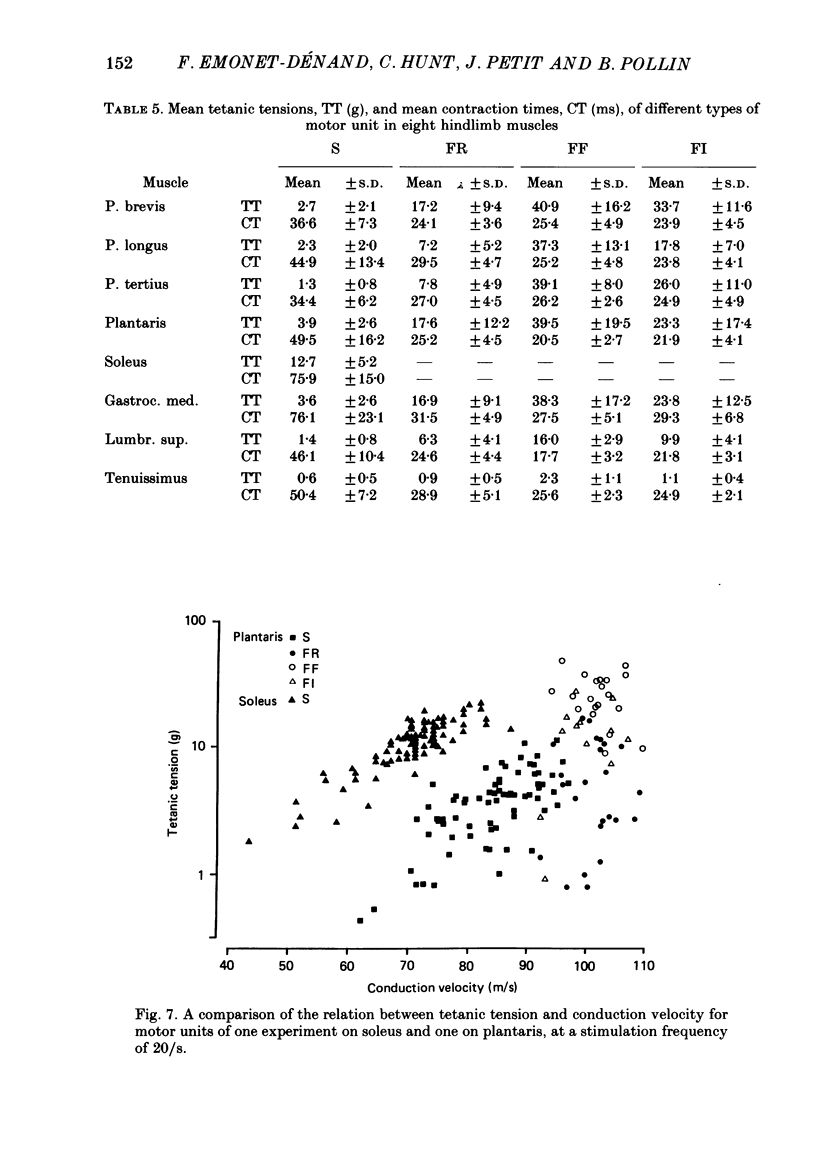
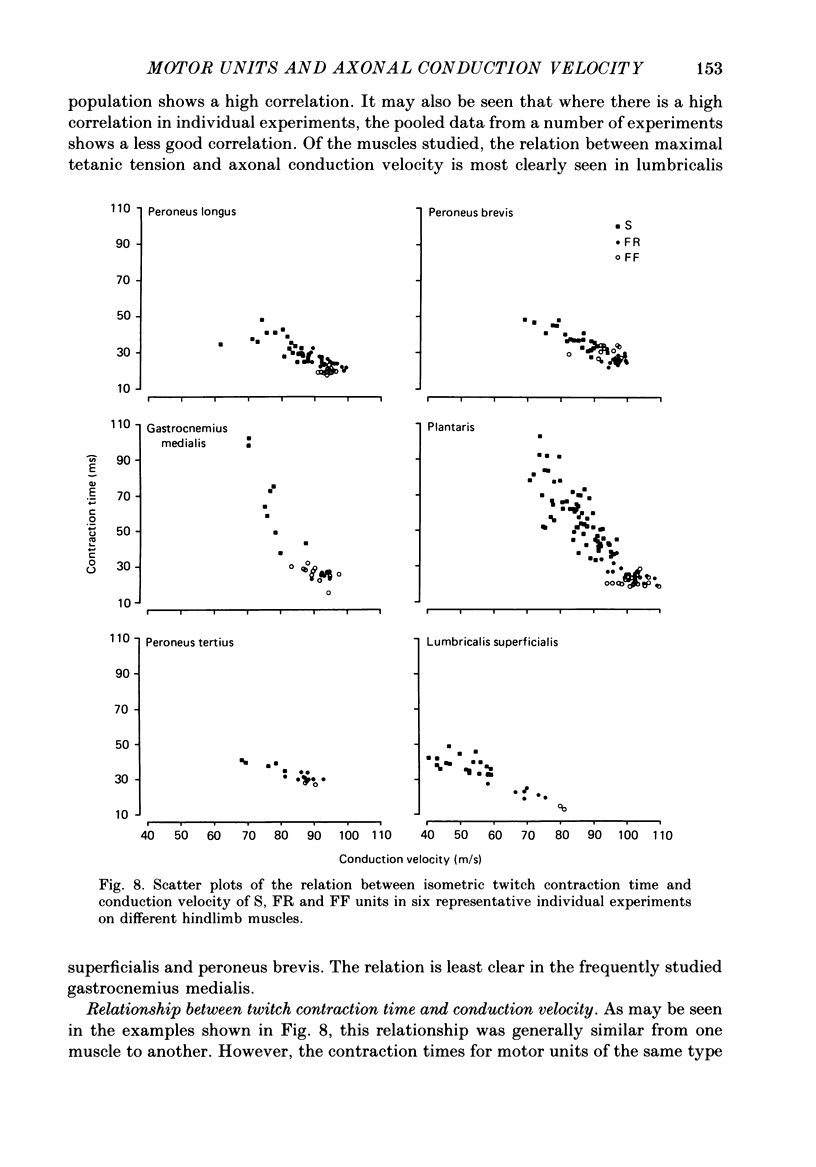
1. A study of motor units to hindlimb muscles of cat has been made, with as complete a sample as possible of the motor axons to an individual muscle. In single experiments as much as 95% of the motor supply to a muscle has been examined. 2. The following muscles have been studied: peroneus brevis, peroneus tertius, peroneus longus, plantaris, gastrocnemius medialis, soleus, tenuissimus and lumbricalis superficialis. 3. Units were identified as slow resistant (S), fast resistant (FR), fast fatigable (FF) and fast intermediate (FI). The proportion of various motor unit types differs from one muscle to another. There is also some variation in the proportions to a given muscle from one animal to another. With the exceptions of soleus, which is entirely slow resistant, and gastrocnemius, which has relatively fewer resistant units, most muscles contain 60% or more of resistant (S and FR) units. 4. The conduction velocity ranges of FF, FR and FI units overlapped. There was little overlap between the conduction velocity ranges of these F units and of S units. 5. In individual experiments there was a strong and significant positive correlation between the logarithm of maximal tetanic tension and axonal conduction velocity in S and in S+FR units. In terms of contractile response the total fatigue-resistant population appeared to be a continuum. The correlation coefficient between maximal tetanic tension and conduction velocity was also high in the totality of units of all types, although within the FF group there appeared to be little or no correlation. In pooled data there was much more scatter and these relations were less clear. This resulted largely from differences in the ranges of axonal conduction velocity for a given motor unit type from one animal to another. 6. There was a highly significant negative correlation between isometric twitch contraction time and axonal conduction velocity in individual experiments. This relationship could also be seen, but less clearly, in pooled data. 7. The possible bases for these relationships are discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. D., Marks W. B., Loeb G. E. The distal hindlimb musculature of the cat. Cutaneous reflexes during locomotion. Exp Brain Res. 1985;58(3):594–603. doi: 10.1007/BF00235875. [DOI] [PubMed] [Google Scholar]
- Adrian E. D. The spread of activity in the tenuissimus muscle of the cat and in other complex muscles. J Physiol. 1925 Sep 4;60(4):301–315. doi: 10.1113/jphysiol.1925.sp002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Bagust J., Knott S., Lewis D. M., Luck J. C., Westerman R. A. Isometric contractions of motor units in a fast twitch muscle of the cat. J Physiol. 1973 May;231(1):87–104. doi: 10.1113/jphysiol.1973.sp010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J. Relationships between motor nerve conduction velocities and motor unit contraction characteristics in a slow twitch muscle of the cat. J Physiol. 1974 Apr;238(2):269–278. doi: 10.1113/jphysiol.1974.sp010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P., Binder M. D., Ruenzel P., Henneman E. Recruitment order of motoneurons in stretch reflexes is highly correlated with their axonal conduction velocity. J Neurophysiol. 1984 Sep;52(3):410–420. doi: 10.1152/jn.1984.52.3.410. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Emonet-Denand F., Joffroy M., Laporte Y. Lack of exclusively fusimotor -axons in flexor and extensor leg muscles of the cat. J Neurophysiol. 1972 Jan;35(1):149–153. doi: 10.1152/jn.1972.35.1.149. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Proske U. Contraction of muscle fibers in two adjacent muscles innervated by branches of the same motor axon. J Neurophysiol. 1971 Jan;34(1):132–138. doi: 10.1152/jn.1971.34.1.132. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J., Zytnicki D. Distribution of physiological types of motor units in the cat peroneus tertius muscle. Exp Brain Res. 1982;48(2):177–184. doi: 10.1007/BF00237213. [DOI] [PubMed] [Google Scholar]
- Jami L., Petit J. Correlation between axonal conduction velocity and tetanic tension of motor units in four muscles of the cat hind limb. Brain Res. 1975 Oct 10;96(1):114–118. doi: 10.1016/0006-8993(75)90581-8. [DOI] [PubMed] [Google Scholar]
- Kernell D., Ducati A., Sjöholm H. Properties of motor units in the first deep lumbrical muscle of the cat's foot. Brain Res. 1975 Nov 7;98(1):37–55. doi: 10.1016/0006-8993(75)90508-9. [DOI] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat's m. peroneus longus. Exp Brain Res. 1983;50(2-3):211–219. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- Kernell D., Sjöholm H. Recruitment and firing rate modulation of motor unit tension in a small muscle of the cat's foot. Brain Res. 1975 Nov 7;98(1):57–72. doi: 10.1016/0006-8993(75)90509-0. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HETEROGENEOUS PALE MUSCLE (M. GASTROCNEMIUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]
- Mizote M. The effect of digital nerve stimulation on recruitment order of motor units in the first deep lumbrical muscle of the cat. Brain Res. 1982 Sep 30;248(2):245–255. doi: 10.1016/0006-8993(82)90582-0. [DOI] [PubMed] [Google Scholar]
- Moshier C. G., Gerlach R. L., Stuart D. G. Soleus and anterior tibial motor units of the cat. Brain Res. 1972 Sep 15;44(1):1–11. doi: 10.1016/0006-8993(72)90361-7. [DOI] [PubMed] [Google Scholar]
- Proske U., Waite P. M. Properties of types of motor units in the medial gastrochemius muscle of the cat. Brain Res. 1974 Feb 15;67(1):89–101. doi: 10.1016/0006-8993(74)90300-x. [DOI] [PubMed] [Google Scholar]
- Proske U., Waite P. M. The relation between tension and axonal conduction velocity for motor units in the medial gastrocnemius muscle of the cat. Exp Brain Res. 1976 Oct 28;26(3):325–328. doi: 10.1007/BF00234936. [DOI] [PubMed] [Google Scholar]
- Reinking R. M., Stephens J. A., Stuart D. G. The motor units of cat medial gastrocnemius: problem of their categorisation on the basis of mechanical properties. Exp Brain Res. 1975 Sep 29;23(3):301–313. doi: 10.1007/BF00239742. [DOI] [PubMed] [Google Scholar]
- Zajac F. E., Faden J. S. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle. J Neurophysiol. 1985 May;53(5):1303–1322. doi: 10.1152/jn.1985.53.5.1303. [DOI] [PubMed] [Google Scholar]


