Abstract
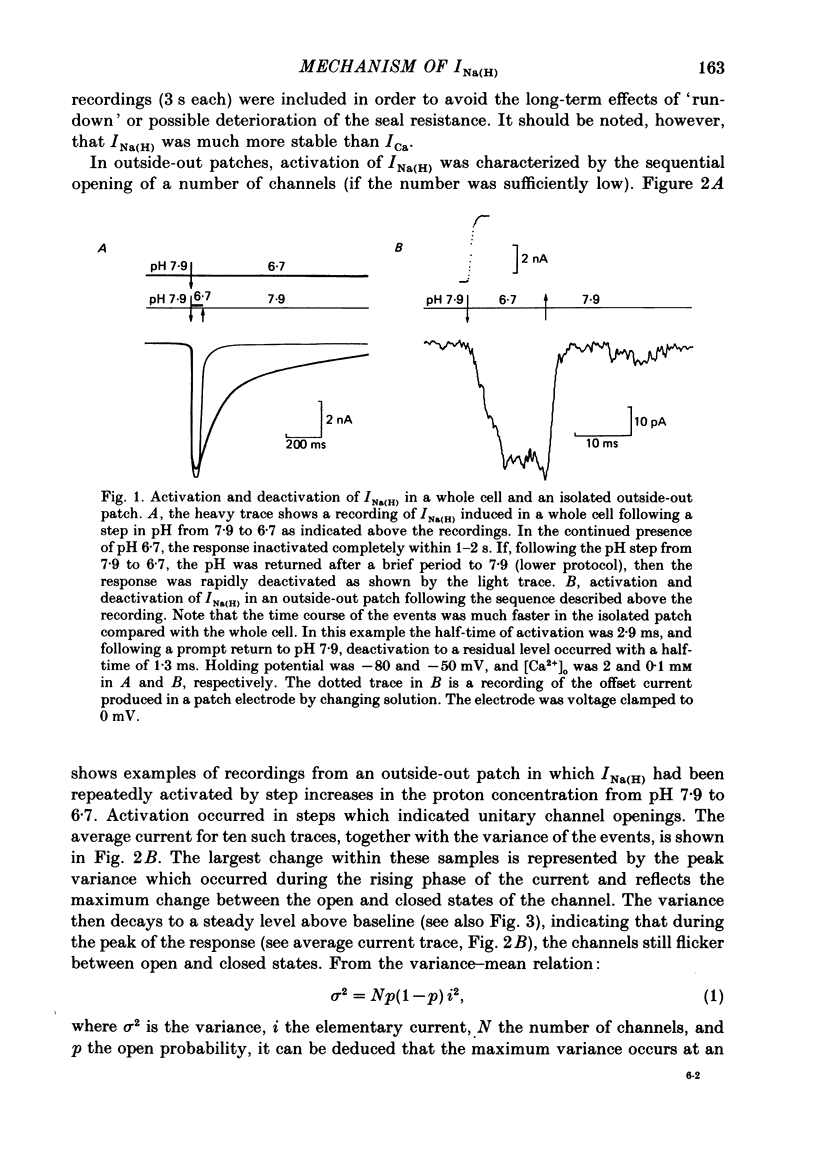
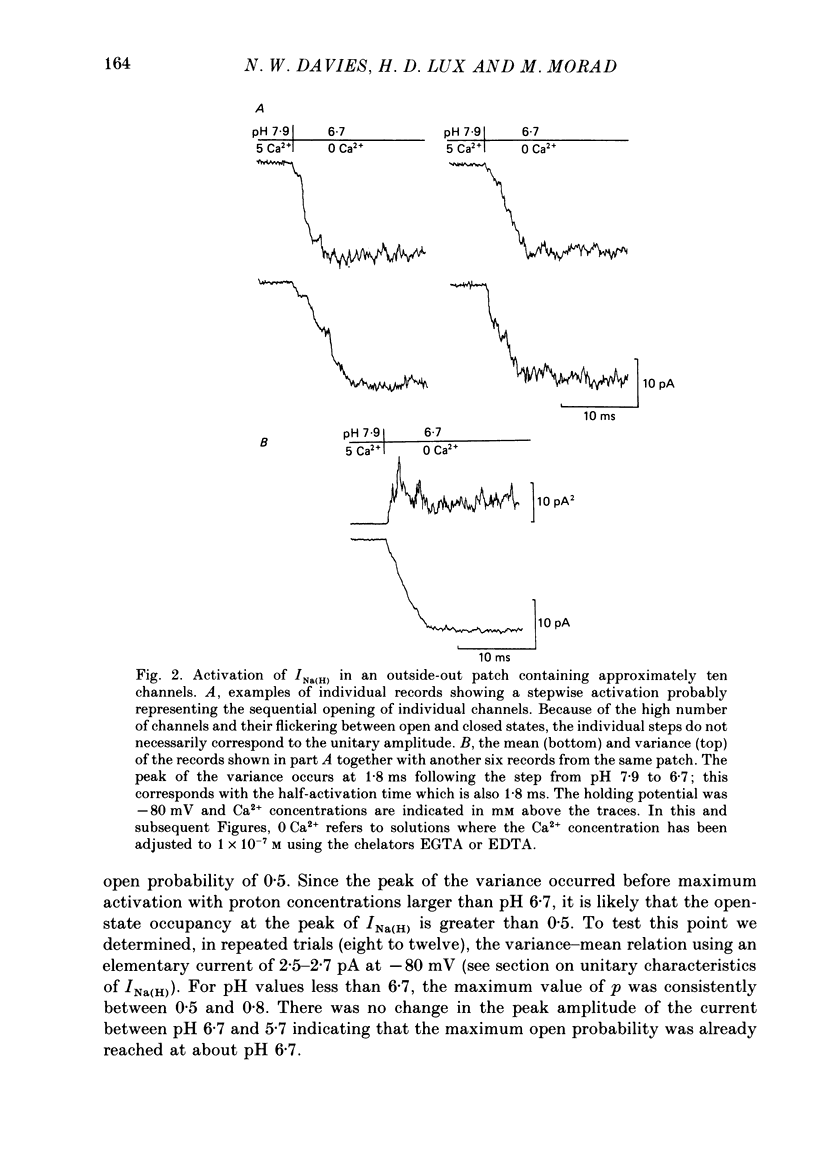
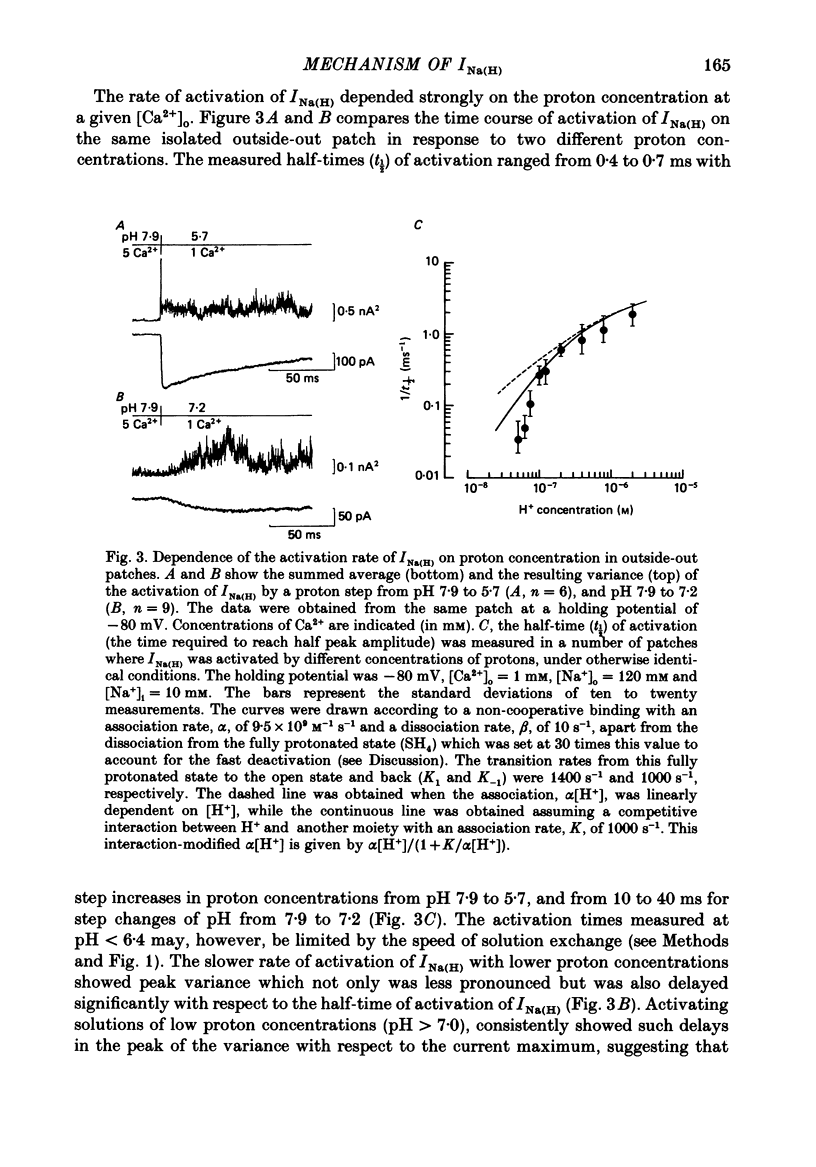
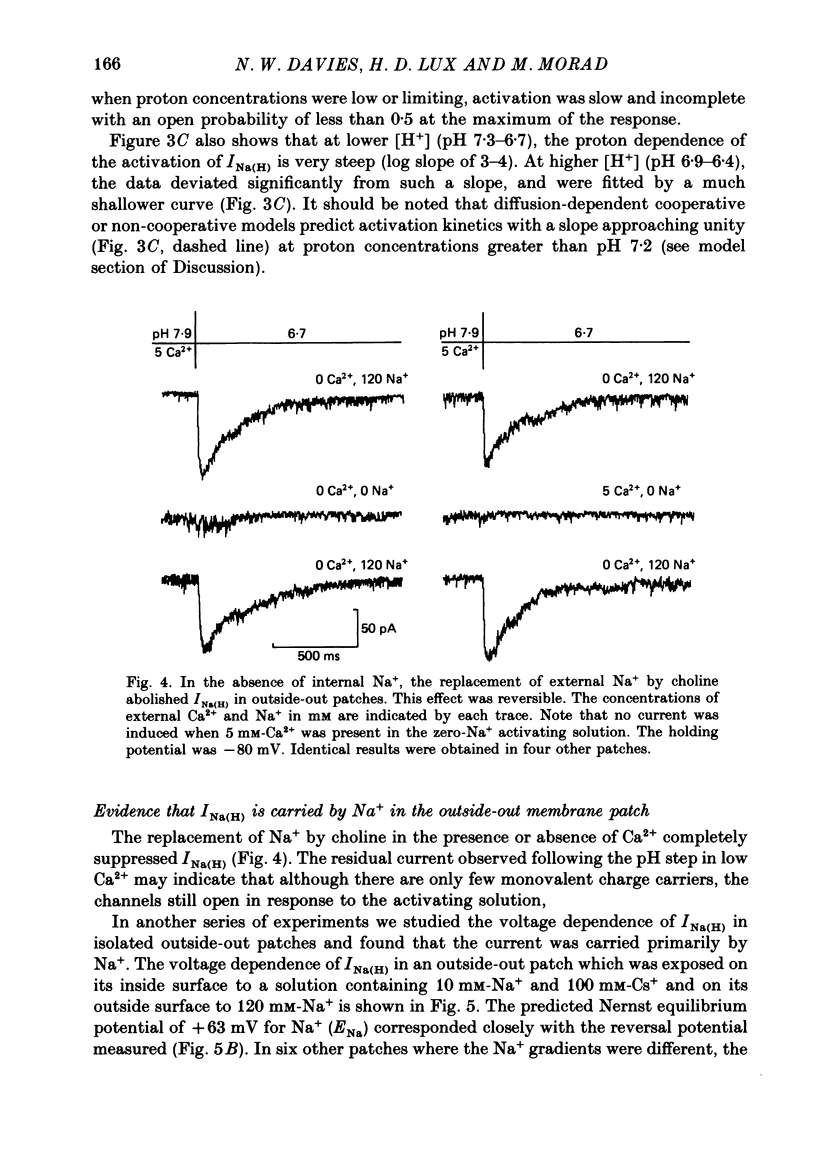
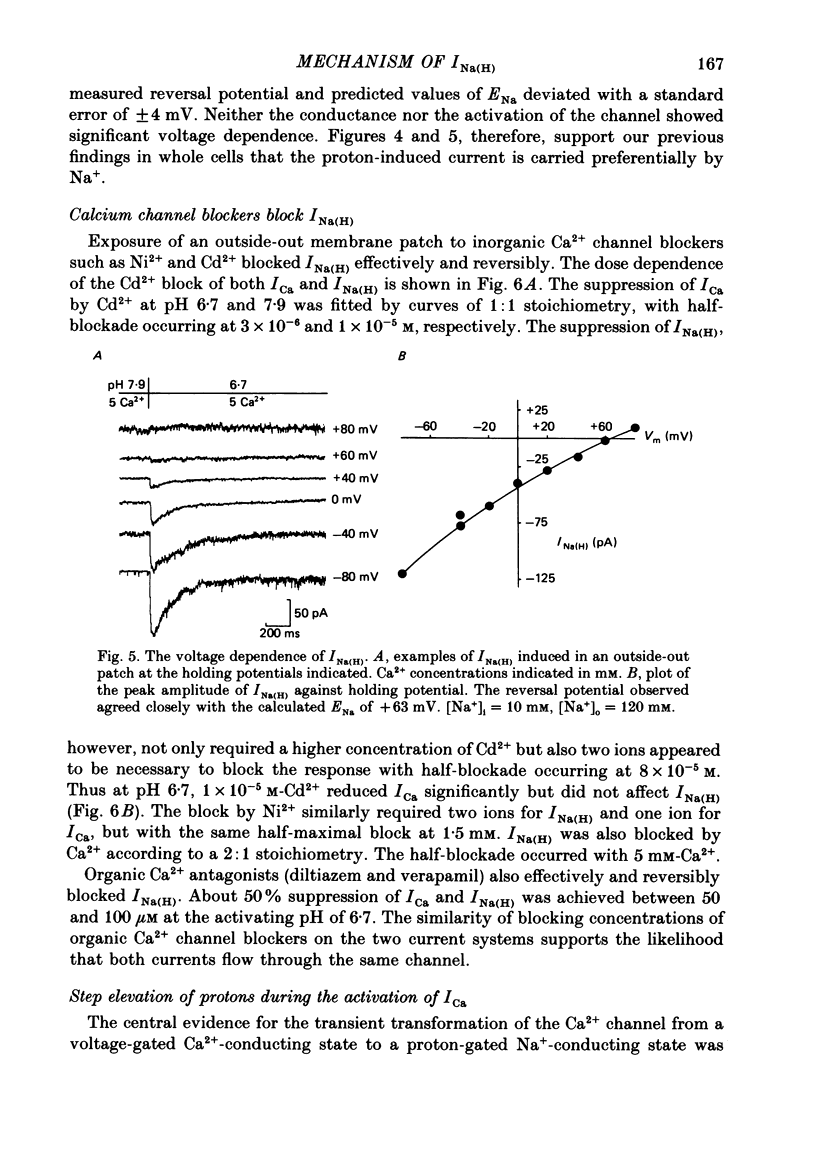
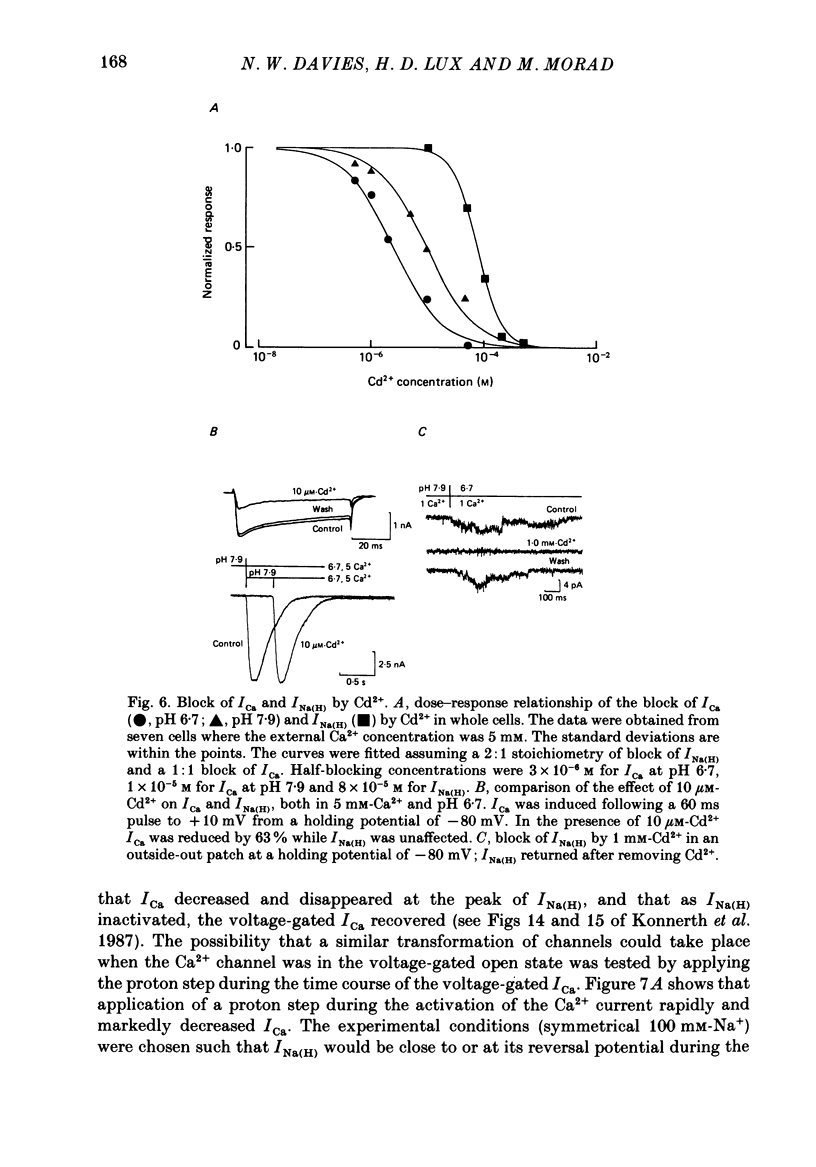
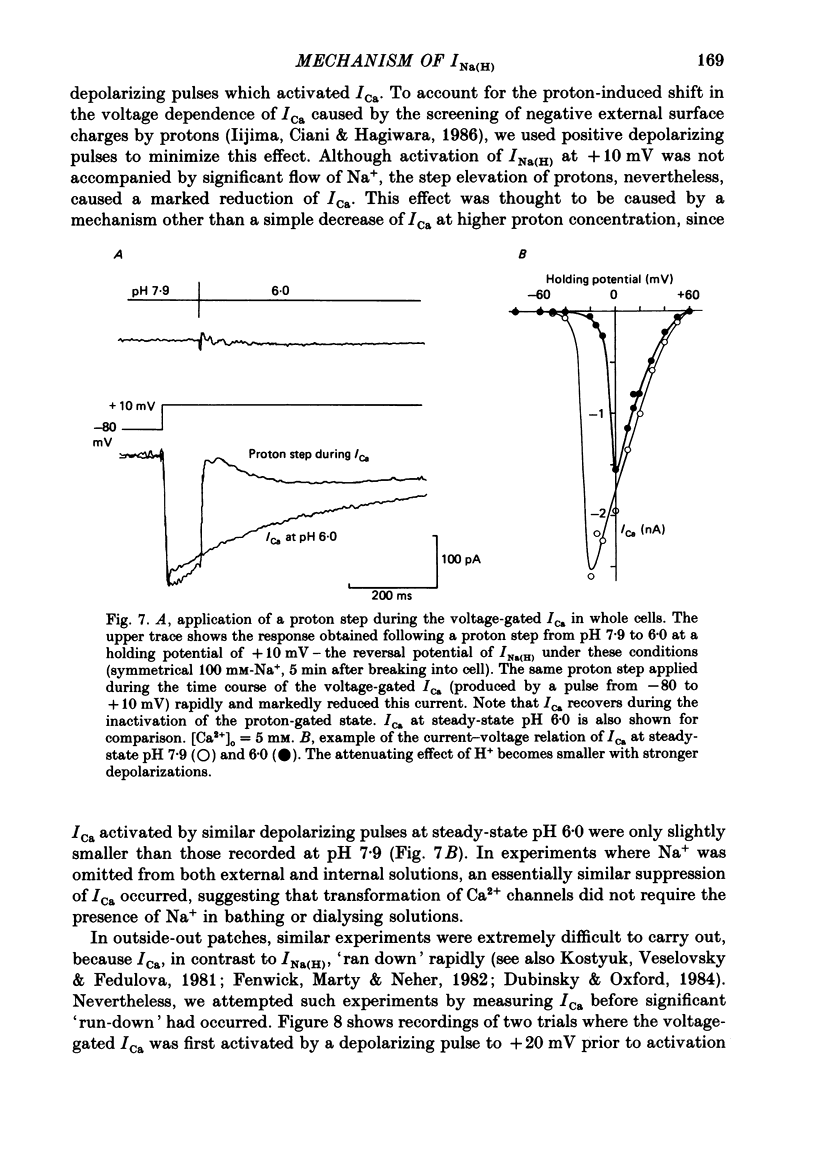
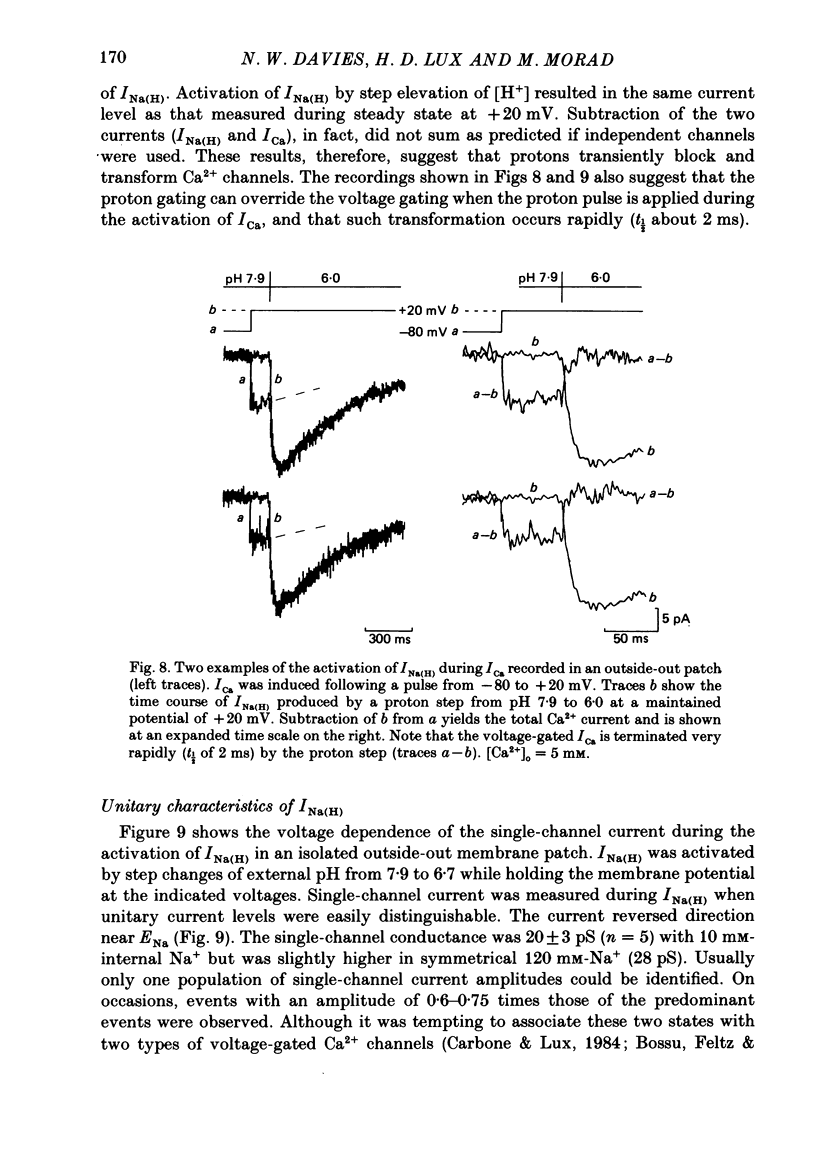
1. In dissociated and cultured 1- to 2-day-old chick dorsal root ganglion cells, and in isolated outside-out membrane patches, a large transient current lasting 1-2 s could be activated upon step increases in [H+]o. The proton-induced current reversed direction at the Na+ equilibrium potential, was abolished completely in the absence of Na+, and was therefore labelled INa(H). 2. To investigate the activation and deactivation kinetics of INa(H) at the single-channel level, we employed isolated membrane patches and a method whereby we could change the external solution in less than 1 ms. 3. In outside-out membrane patches, INa(H) was fully activated within 2 ms between pH 6.7 and 5.7. Half-times of activation decreased with increasing [H+]o. The calculated association rate constant was 9.5 x 10(9) M-1 s-1. 4. Deactivation of INa(H), following a step reduction in [H+]o, occurred with half-times of within 1.3-2 ms. 5. In the continued presence of an activating solution (pH 6.7 and 1 mM-Ca2+), INa(H) inactivated slowly, with a time constant of about 300 ms. 6. Inactivation showed a limited dependence on [Ca2+]o. The time constant of inactivation increased from about 300-500 ms as [Ca2+]o was decreased from 5 to 0.1 mM. Further decrease in [Ca2+]o did not significantly increase the time course of inactivation. Increases in [Ca2+]i from 10(-9) to 10(-3) M had no effect on the activation or inactivation kinetics of INa(H). 7. Conditioning proton concentrations which by themselves failed to activate any channel openings, partially inactivated INa(H). 8. Recovery from inactivation appeared to follow a time course similar to that of inactivation itself. 9. INa(H) could not be activated in inside-out patches. A step increase in proton concentration outside a cell-attached patch was also ineffective at producing INa(H) in the patch. Intracellular pH between 7.9 and 6.7 had no effect on the activation or inactivation of INa(H). 10. The activation and inactivation kinetics were not significantly voltage dependent. 11. The single-channel conductance associated with the activation of INa(H) was 28 pS in symmetrical 120 mM-NaCl solutions and remained constant throughout the time course of INa(H). 12. During activation of the voltage-gated calcium current, ICa, a step increase in proton concentration caused a rapid (ca. 2 ms) suppression of ICa which was more than that predicted from the steady-state effects of H+ on ICa. This effect was independent of [Na+] and the direction of INa(H).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



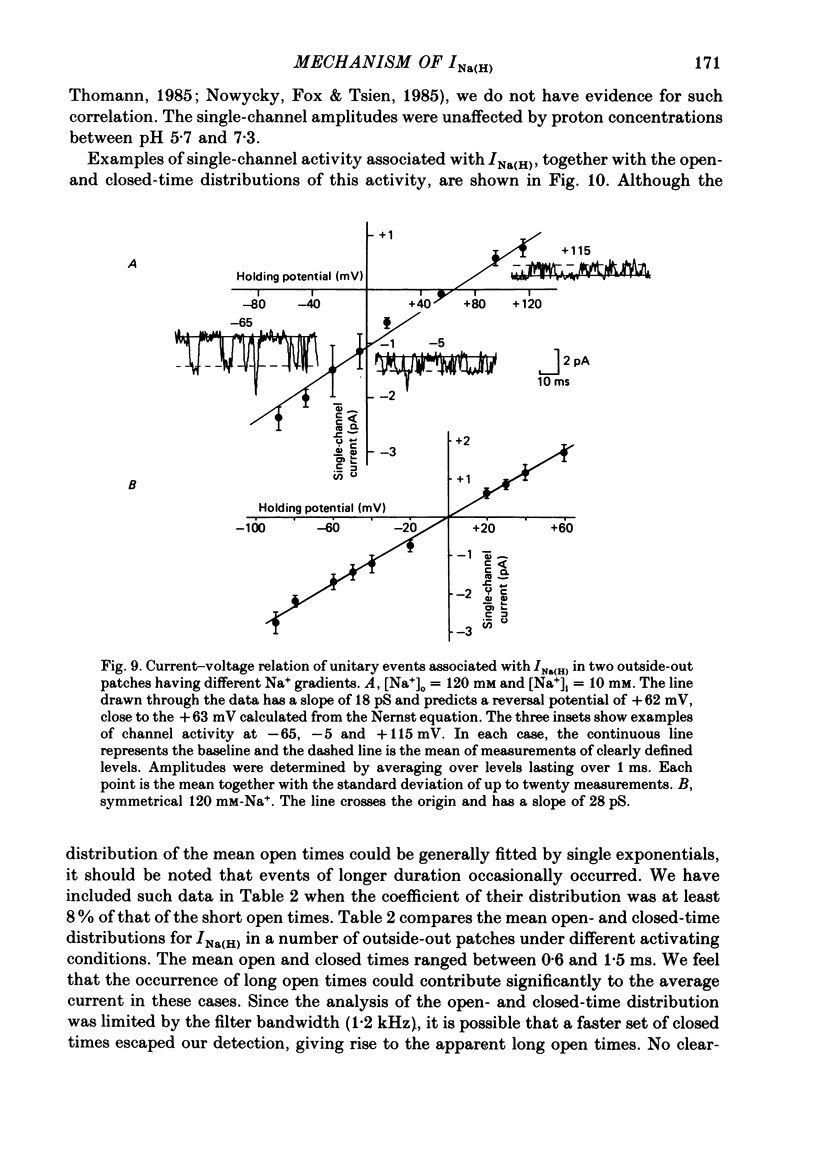
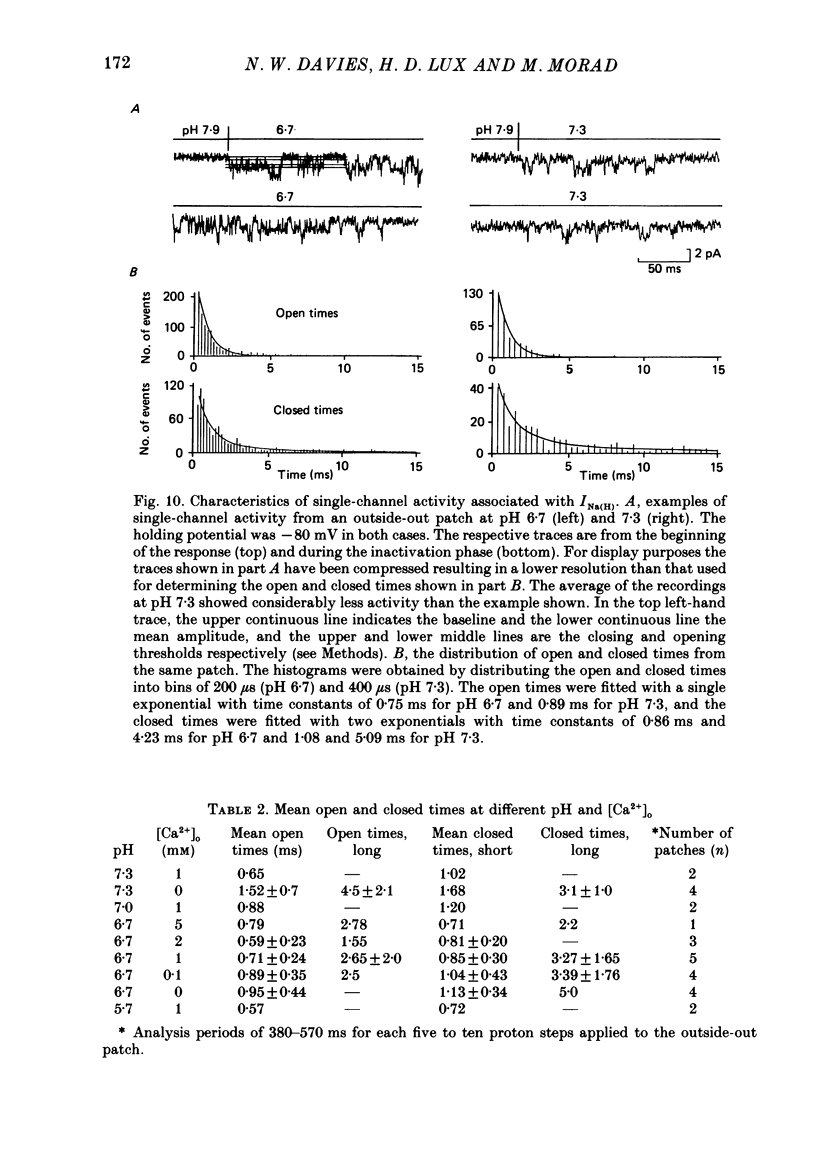

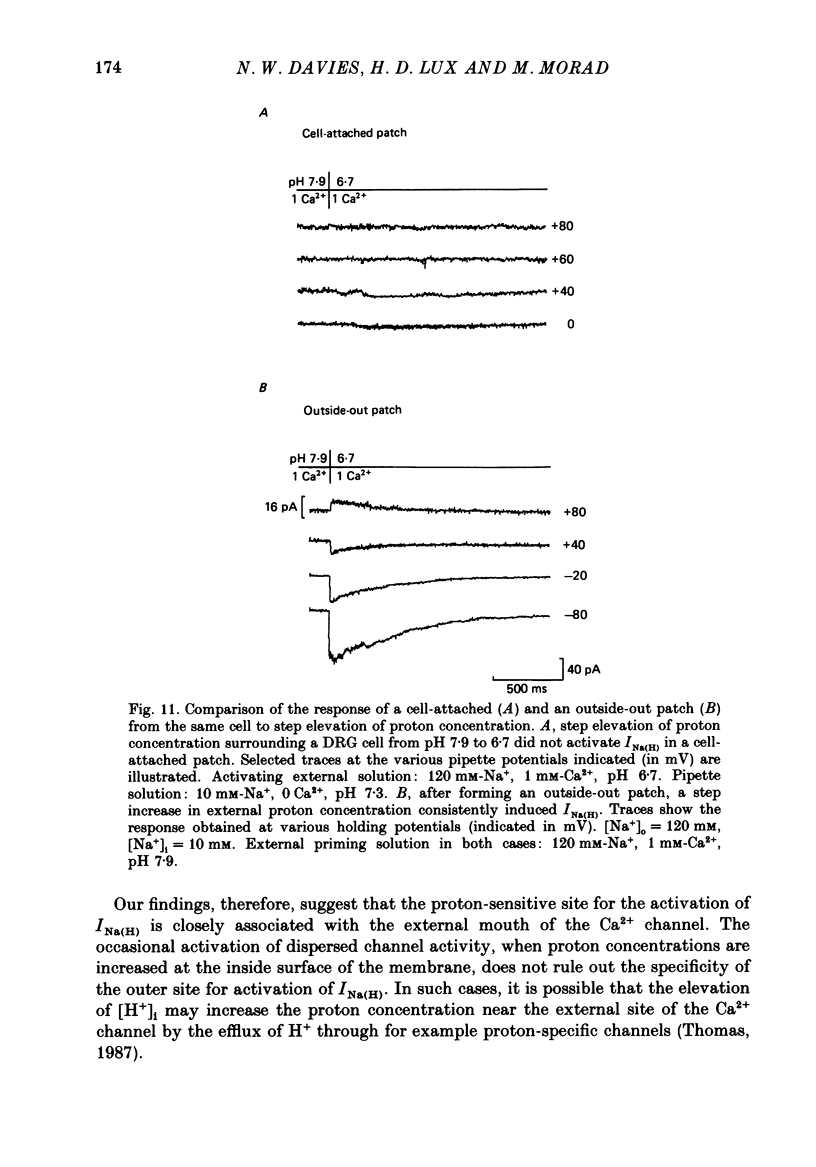
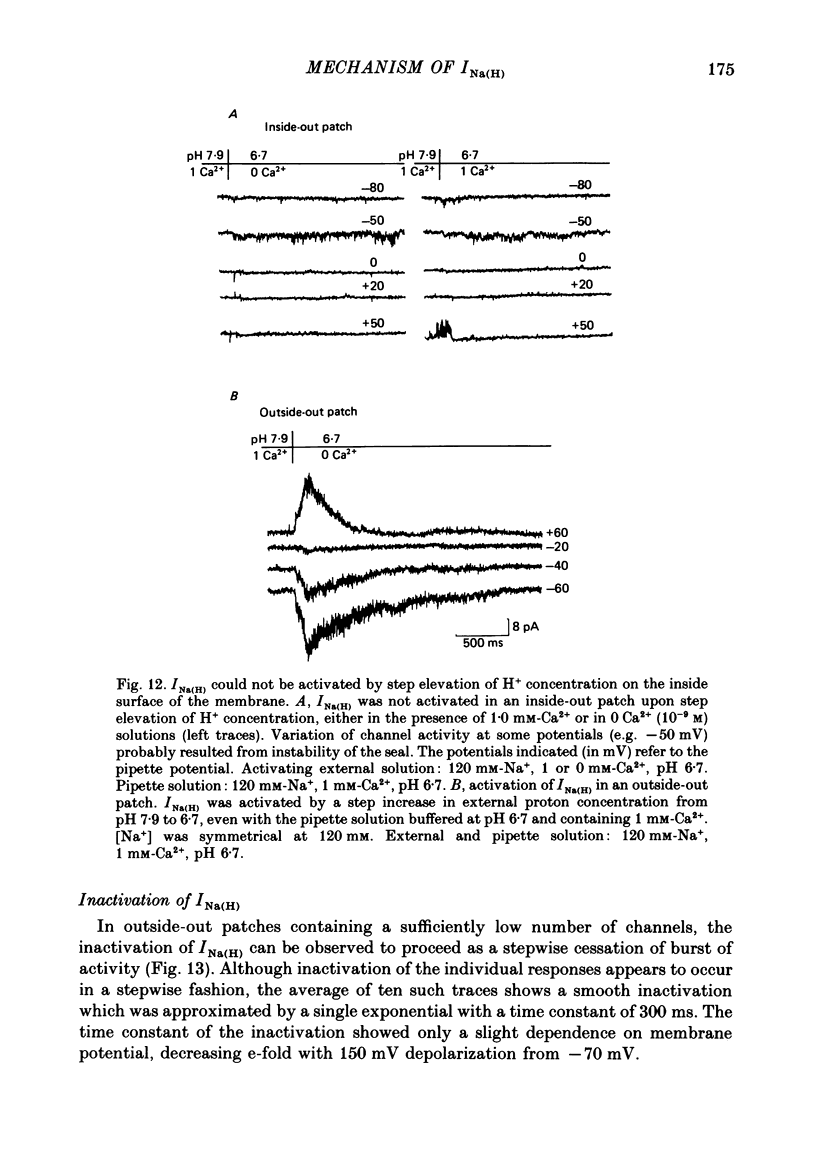






Selected References
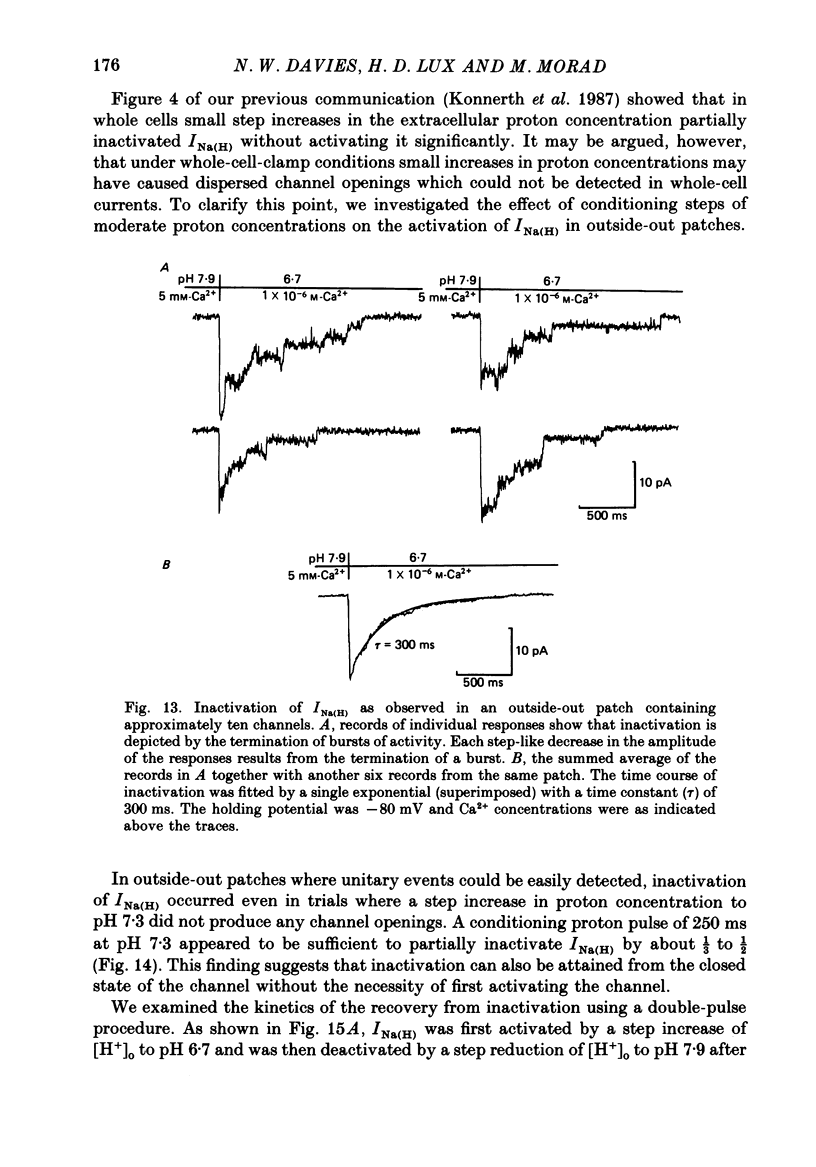
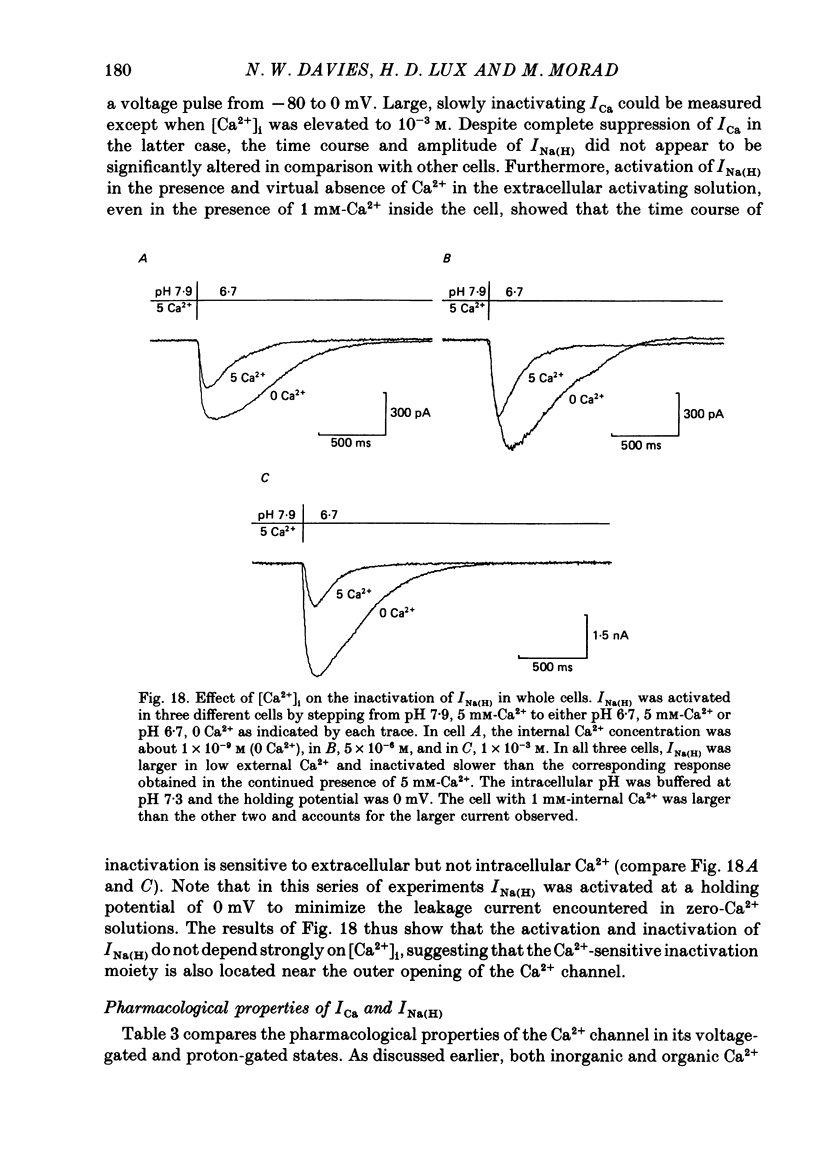
These references are in PubMed. This may not be the complete list of references from this article.
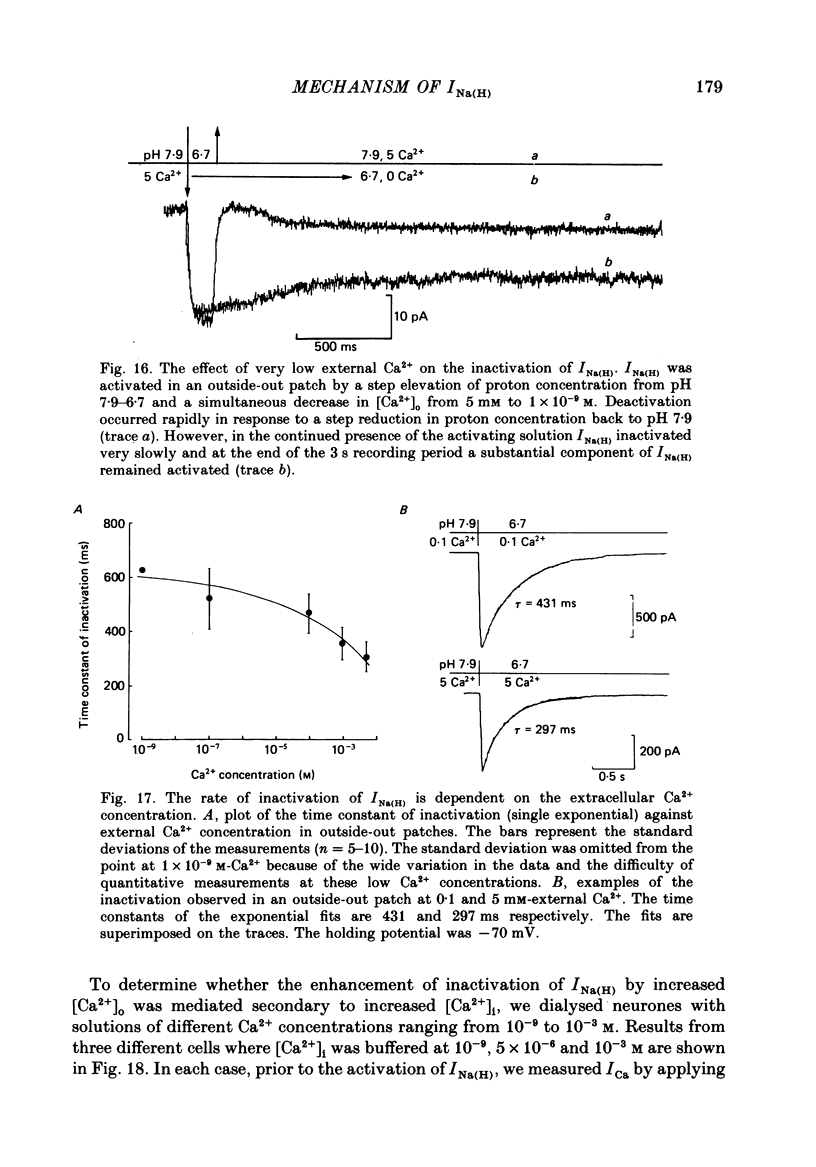
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
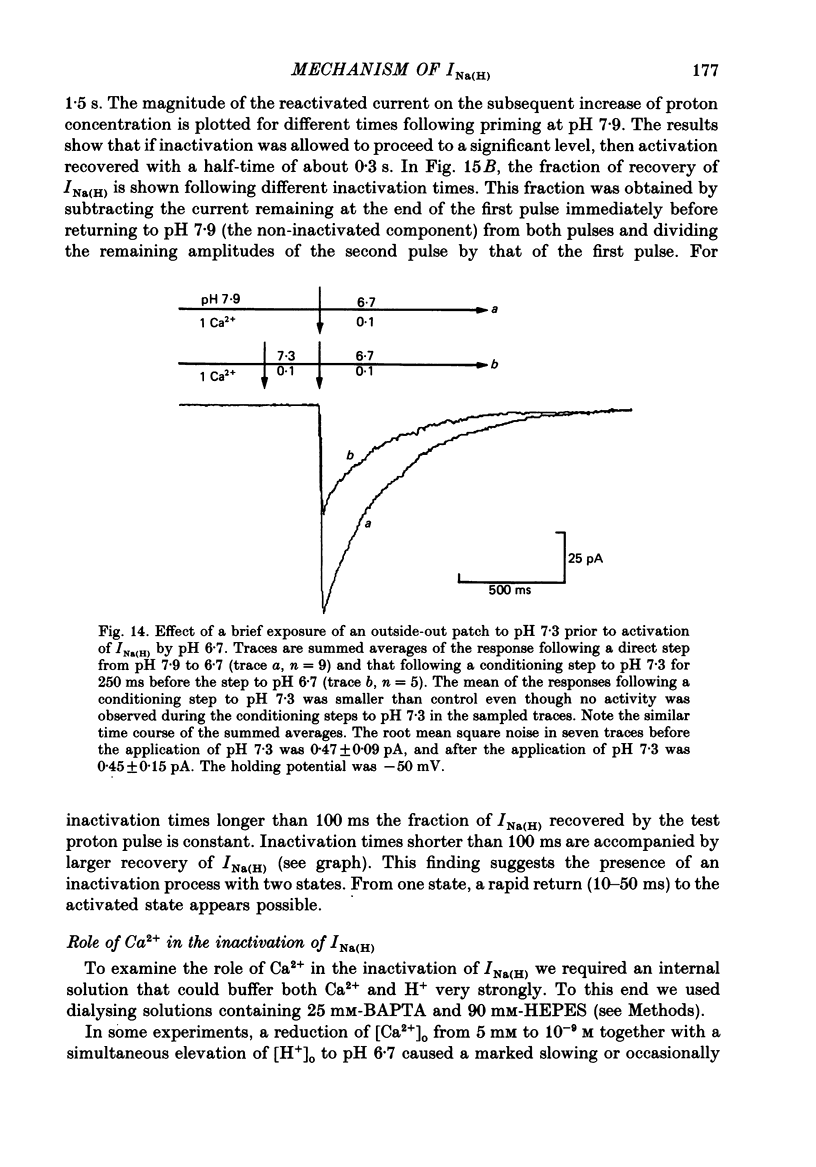
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
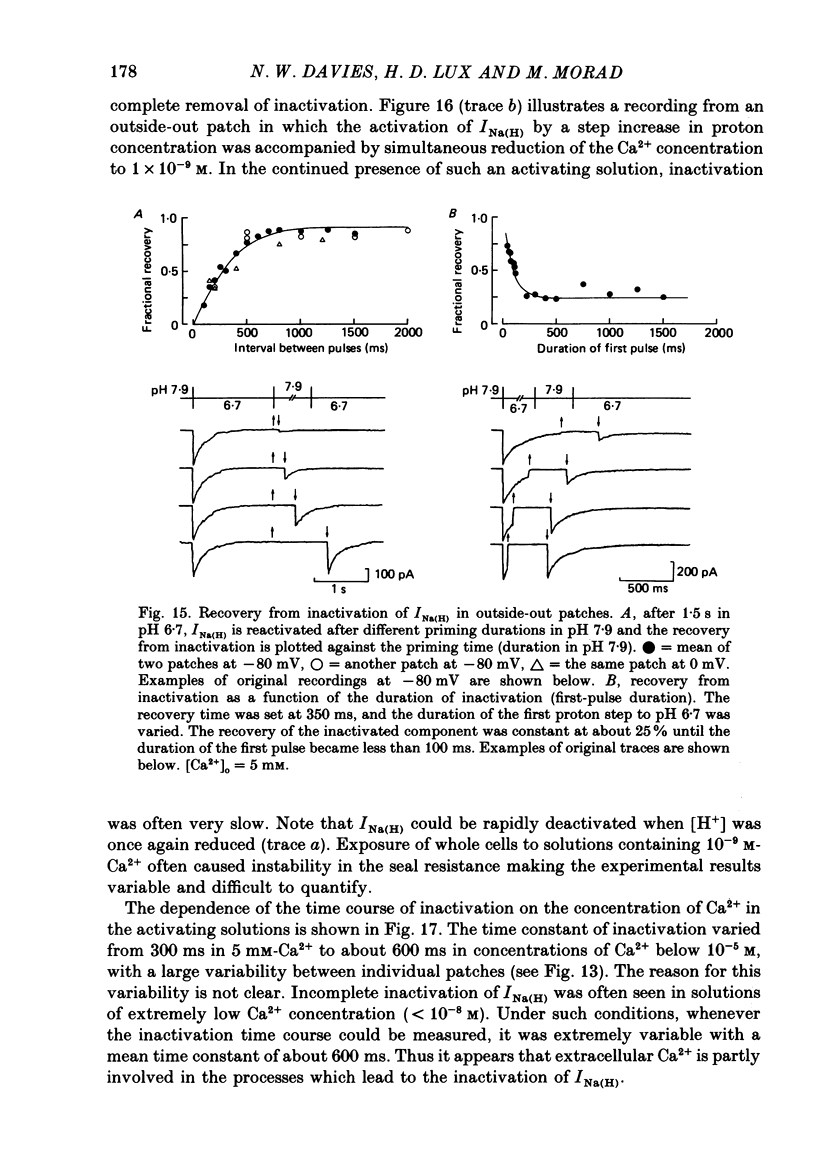
- Byerly L., Meech R., Moody W., Jr Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984 Jun;351:199–216. doi: 10.1113/jphysiol.1984.sp015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Membrane currents of internally perfused neurones of the snail, Lymnaea stagnalis, at low intracellular pH. J Physiol. 1986 Jul;376:477–491. doi: 10.1113/jphysiol.1986.sp016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
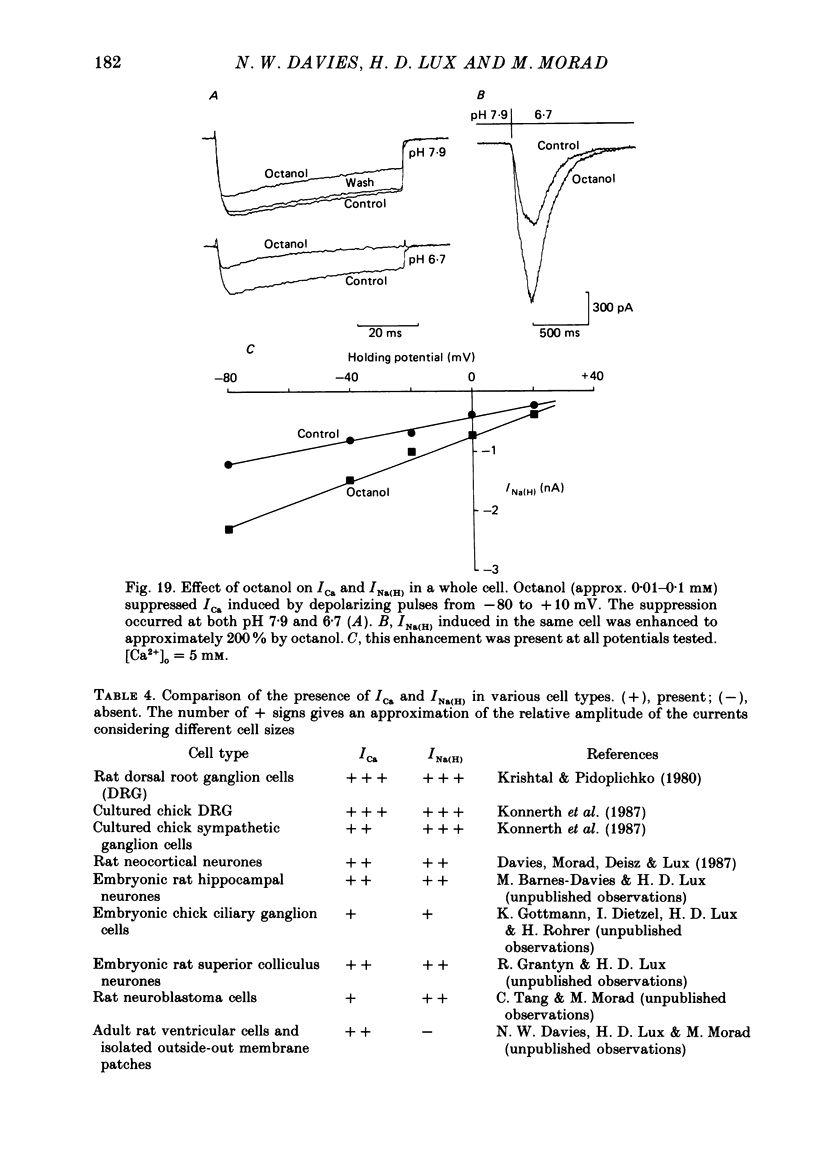
- Carbone E., Lux H. D. Single low-voltage-activated calcium channels in chick and rat sensory neurones. J Physiol. 1987 May;386:571–601. doi: 10.1113/jphysiol.1987.sp016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Dubinsky J. M., Oxford G. S. Ionic currents in two strains of rat anterior pituitary tumor cells. J Gen Physiol. 1984 Mar;83(3):309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J., Heinemann U., Lux H. D. Step reductions in extracellular Ca2+ activate a transient inward current in chick dorsal root ganglion cells. Biophys J. 1986 Oct;50(4):753–757. doi: 10.1016/S0006-3495(86)83515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Fleckenstein A. Inhibition of the slow inward current by nifedipine in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jul;298(3):267–272. doi: 10.1007/BF00500899. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6(12):2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Schäfer K., Lux H. D. Calcium channel current inactivation is selectively modulated by menthol. Neurosci Lett. 1986 Jul 11;68(1):23–28. doi: 10.1016/0304-3940(86)90223-5. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Meech R. W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982 Oct 28;299(5886):826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]


