Abstract
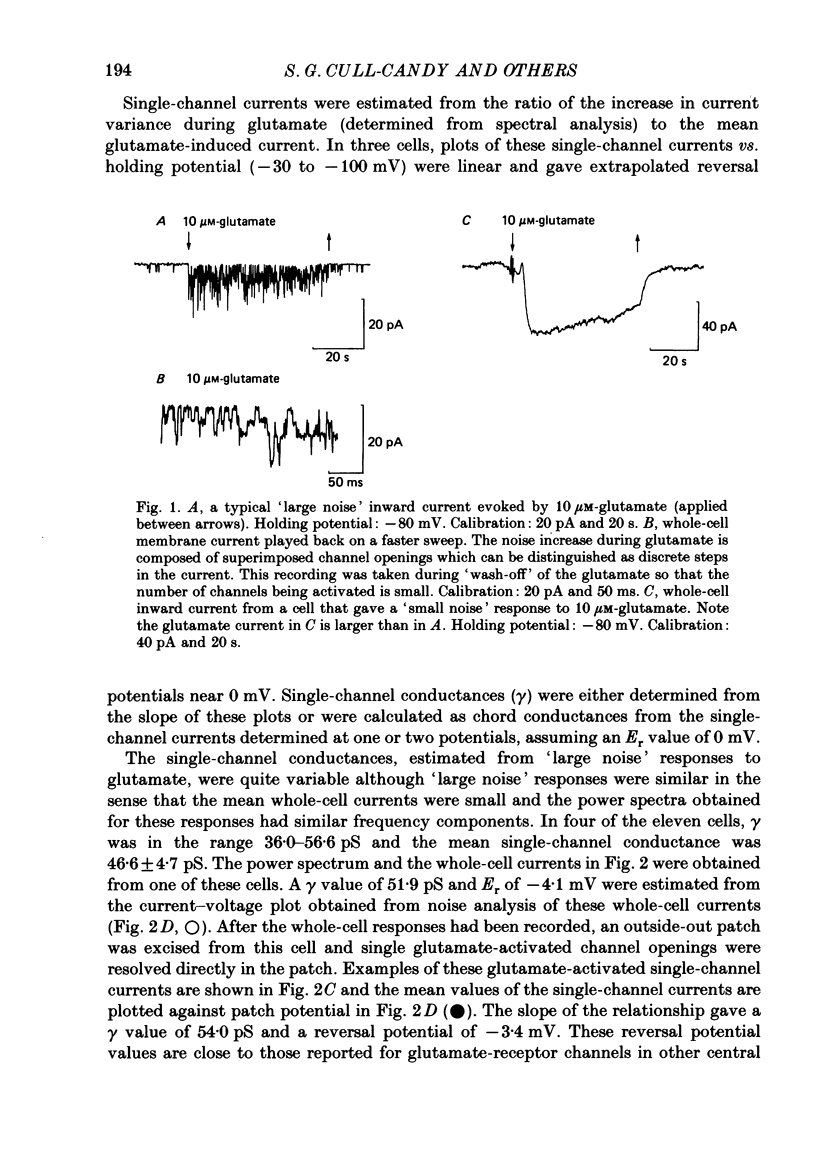
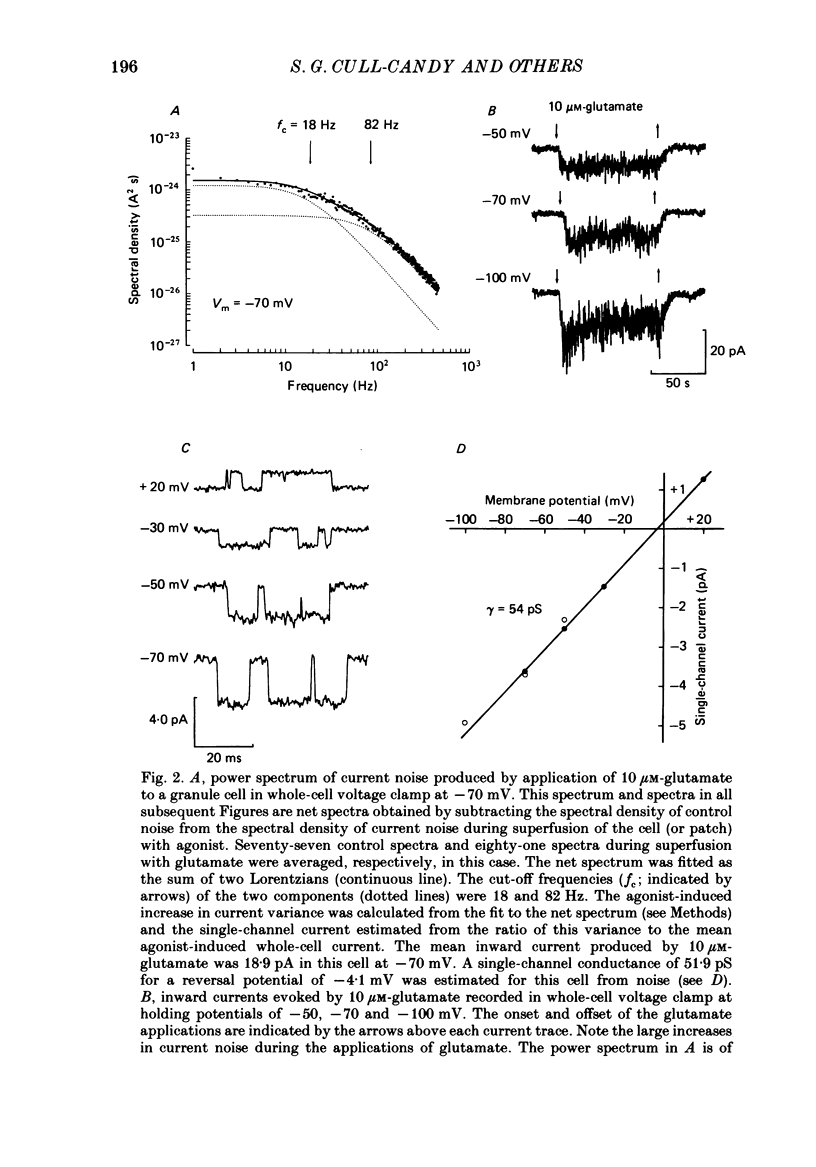
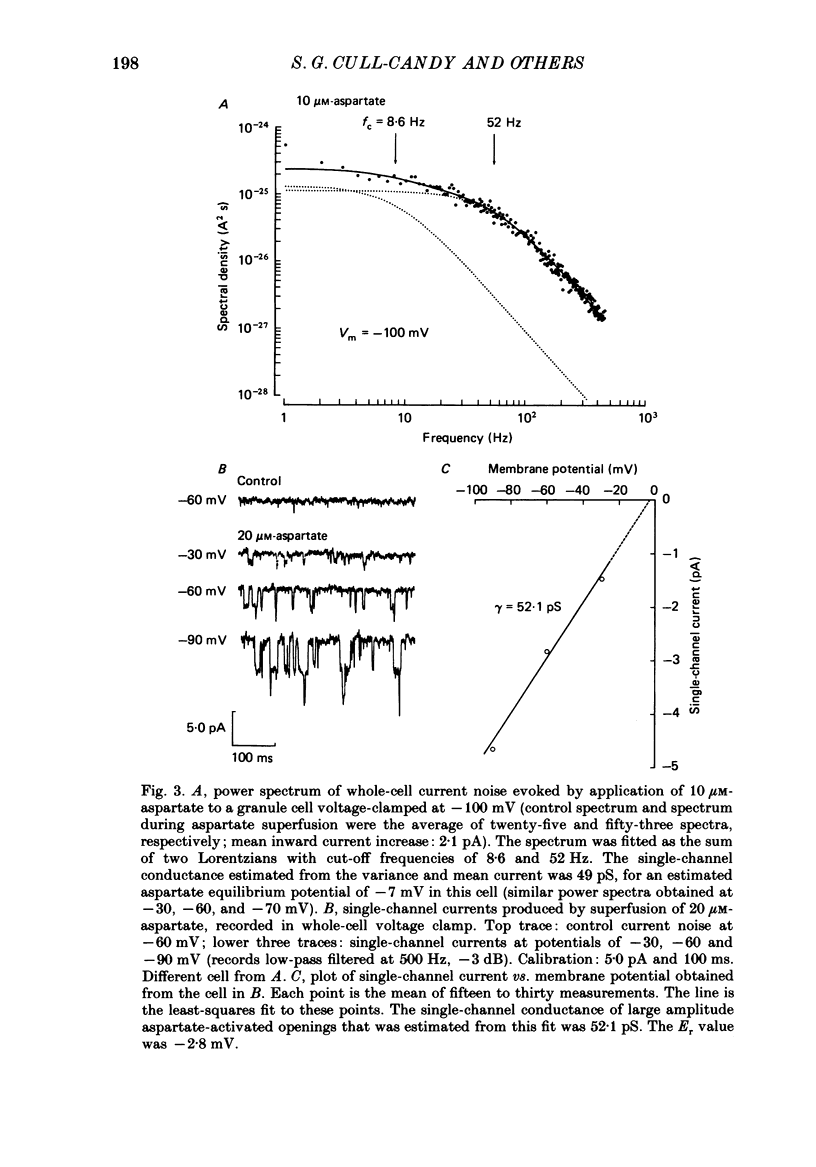
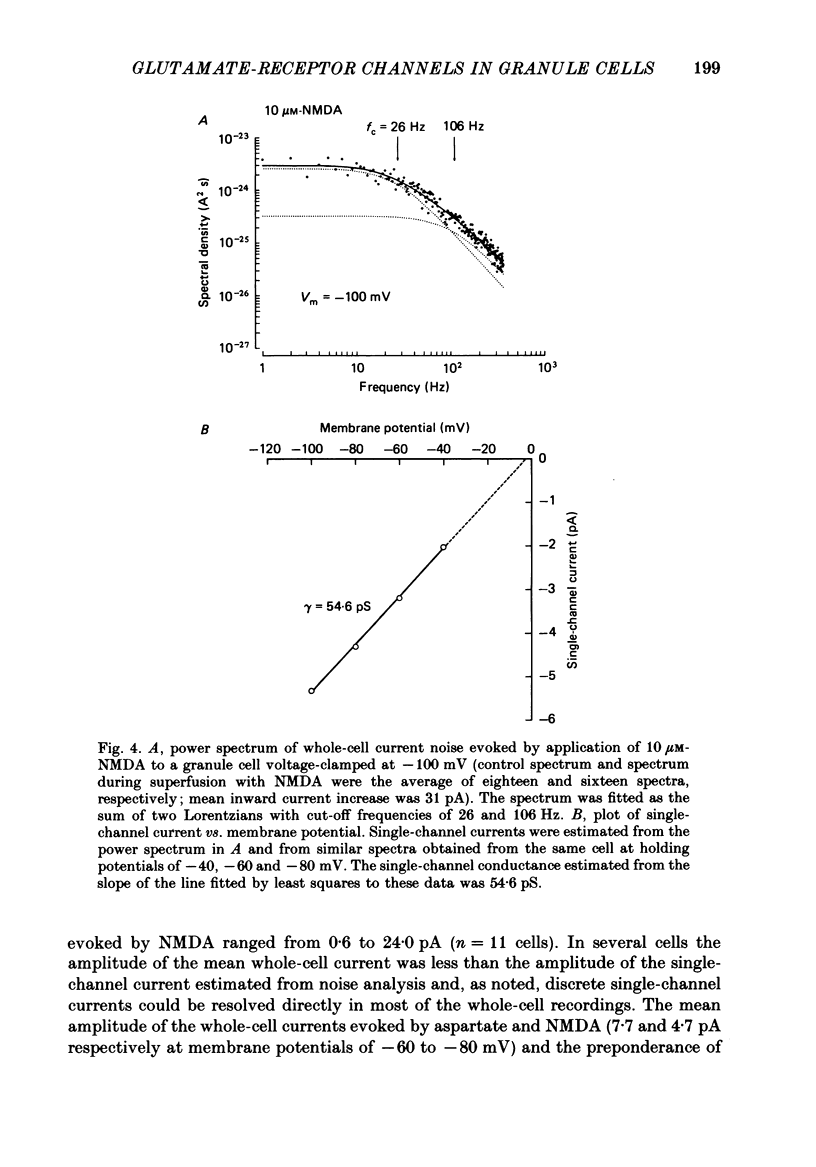
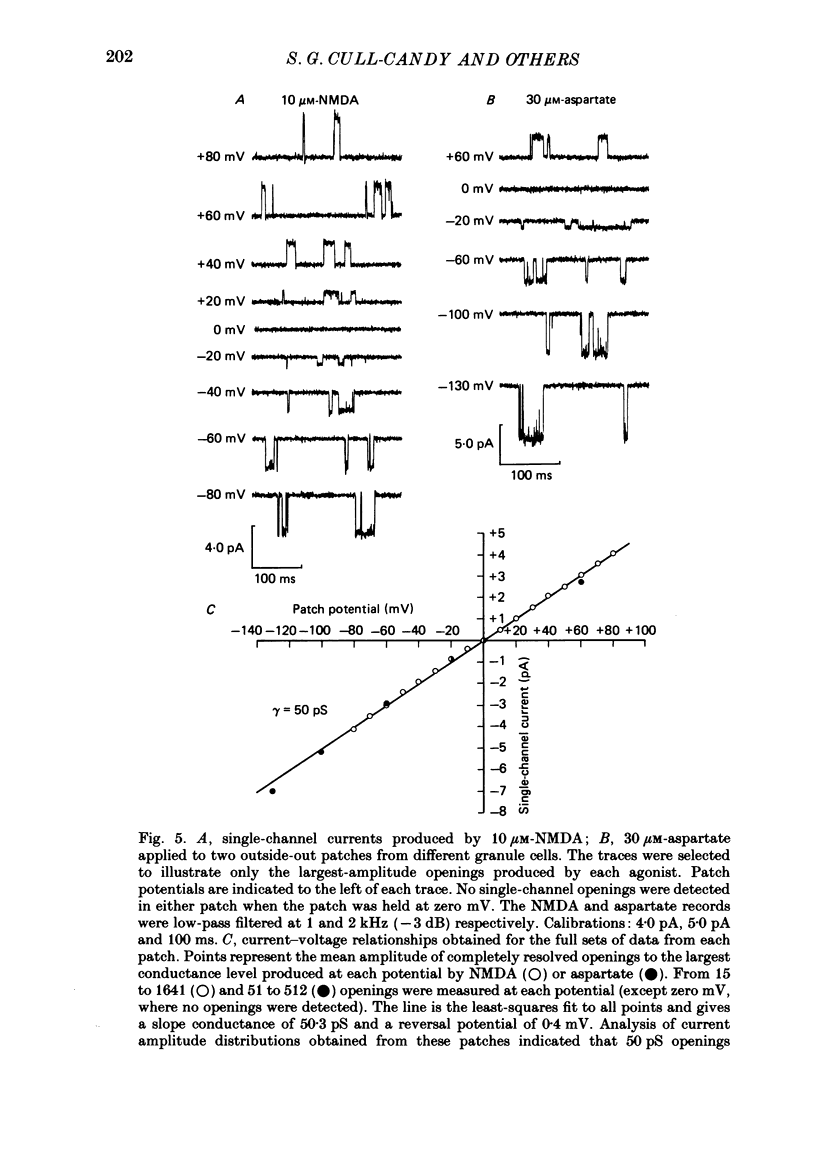
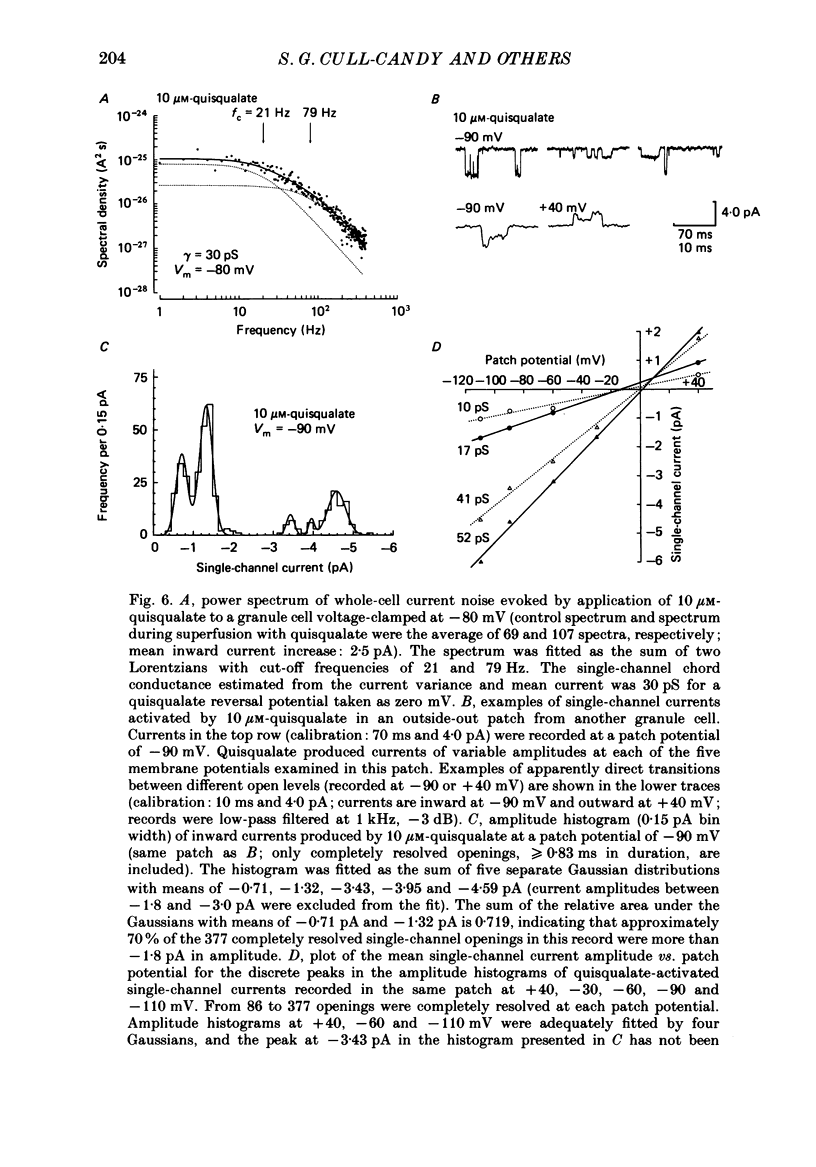
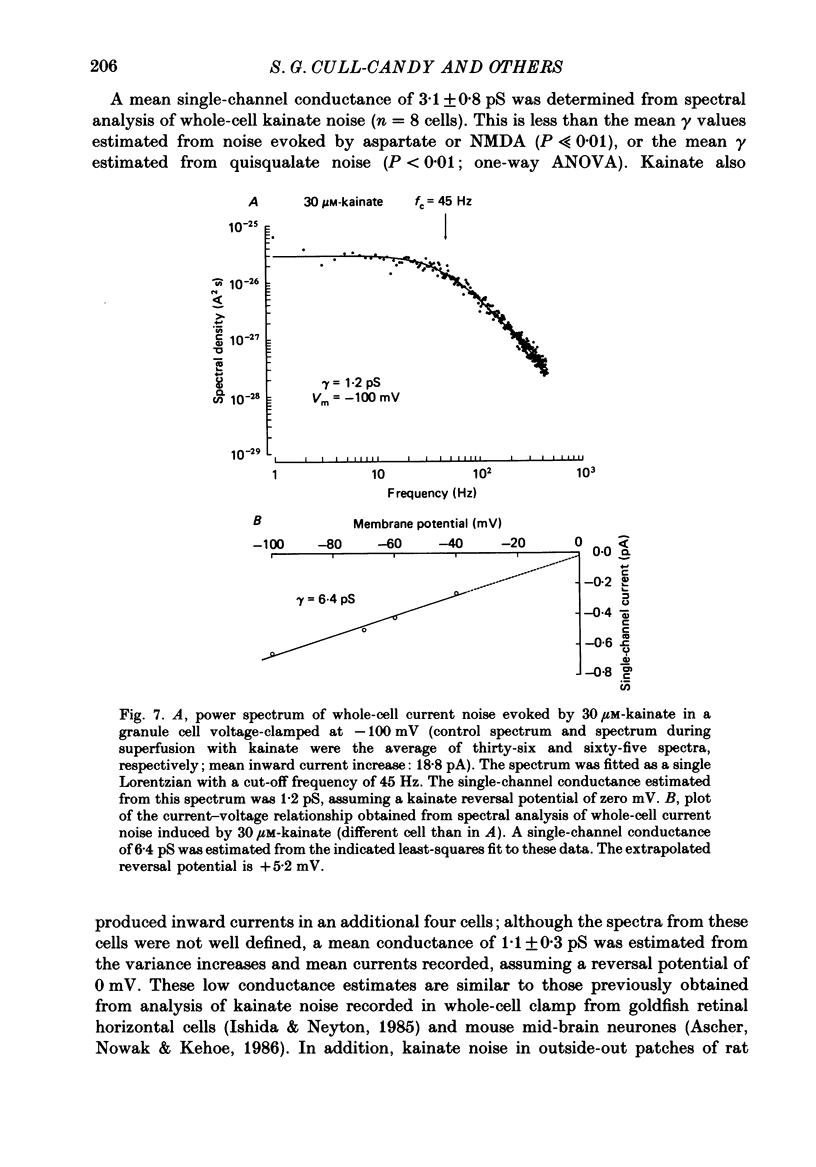
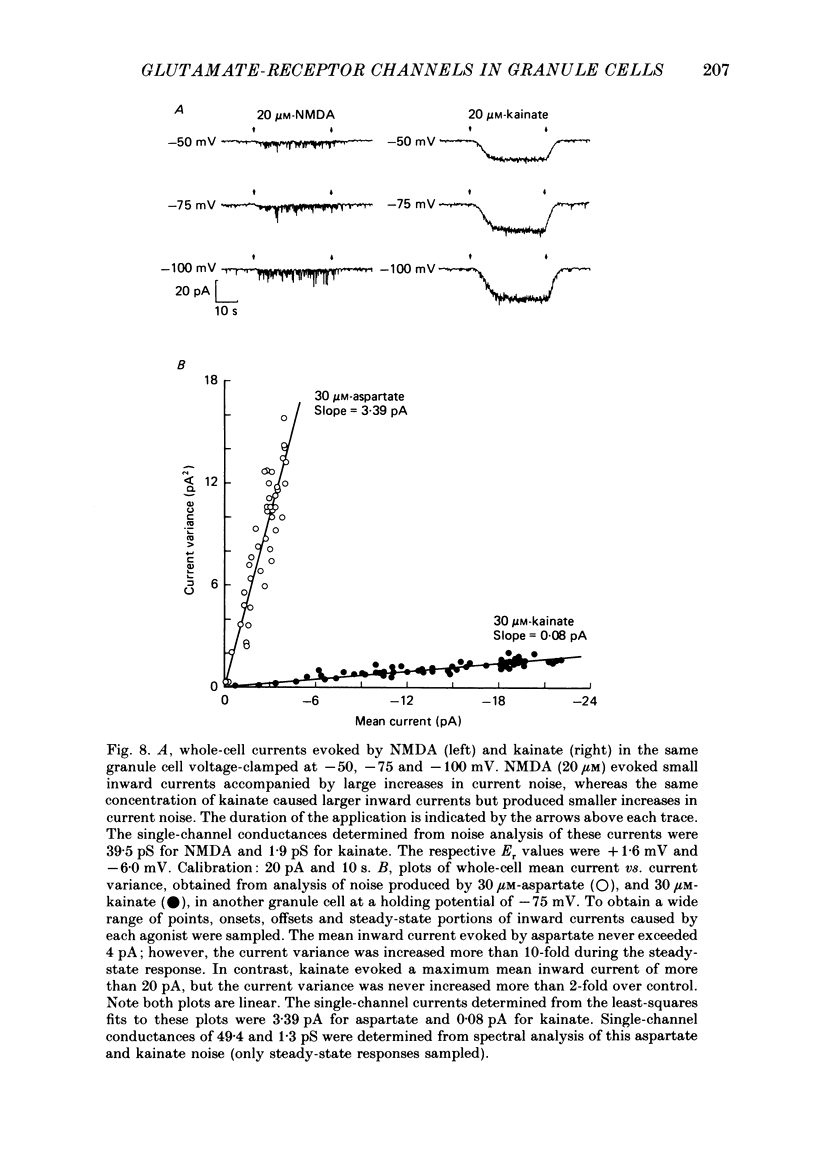
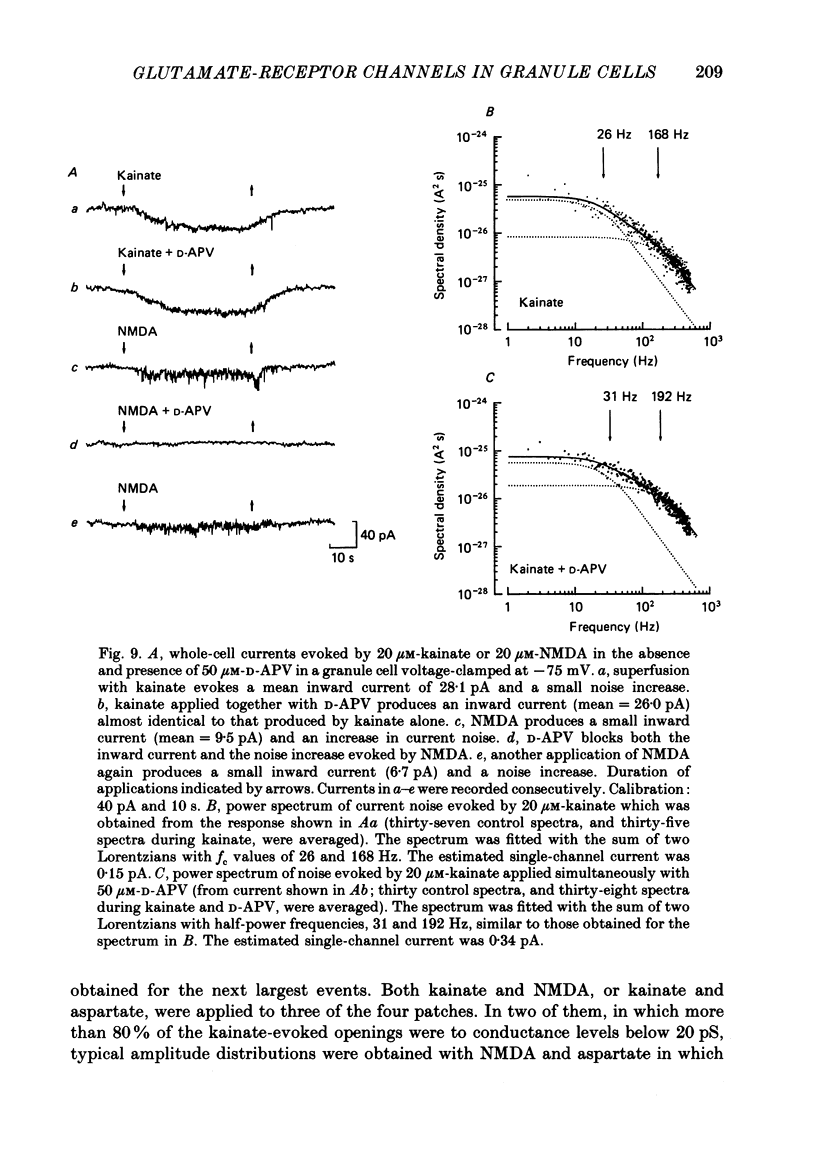
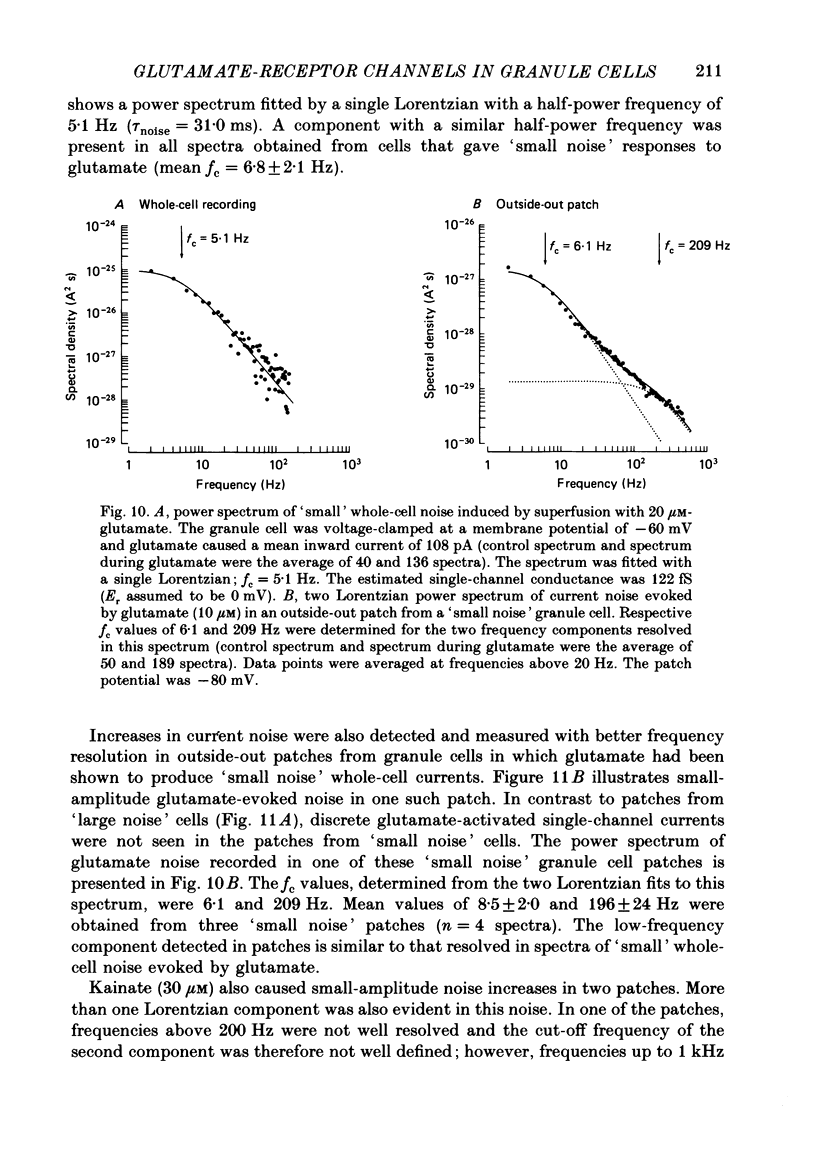
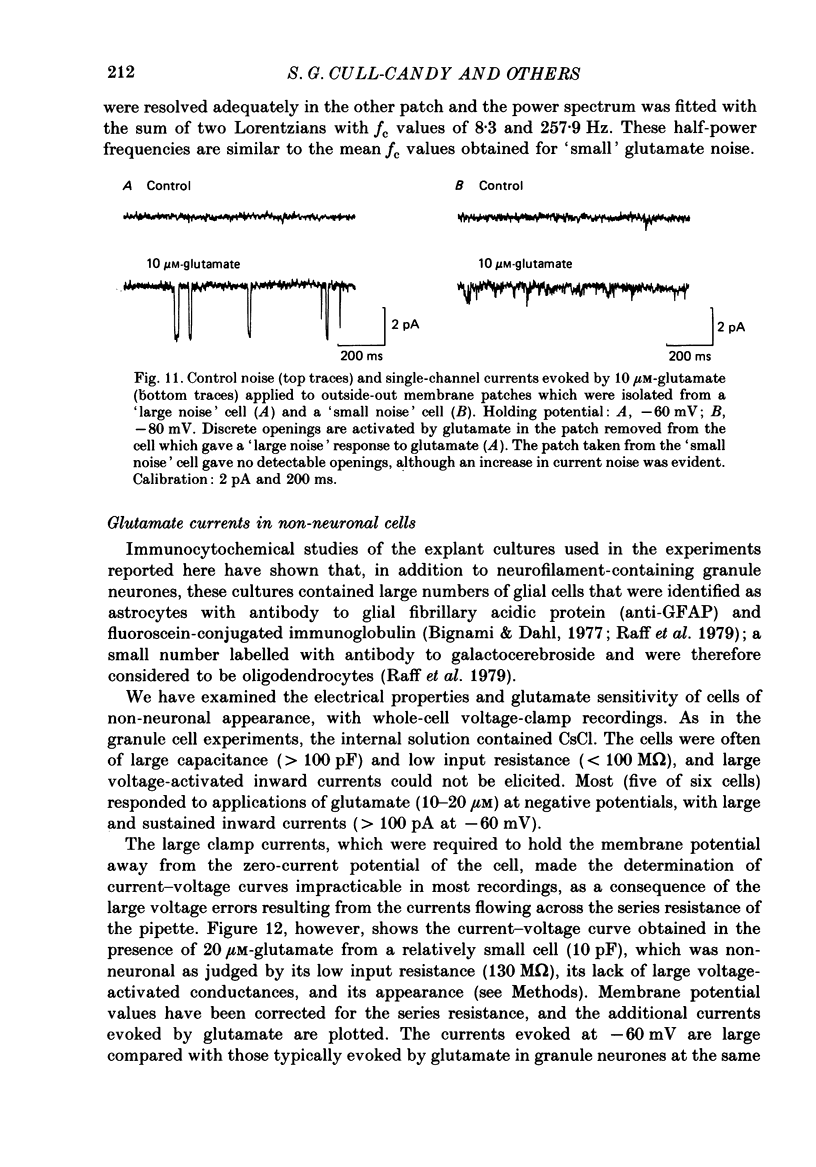
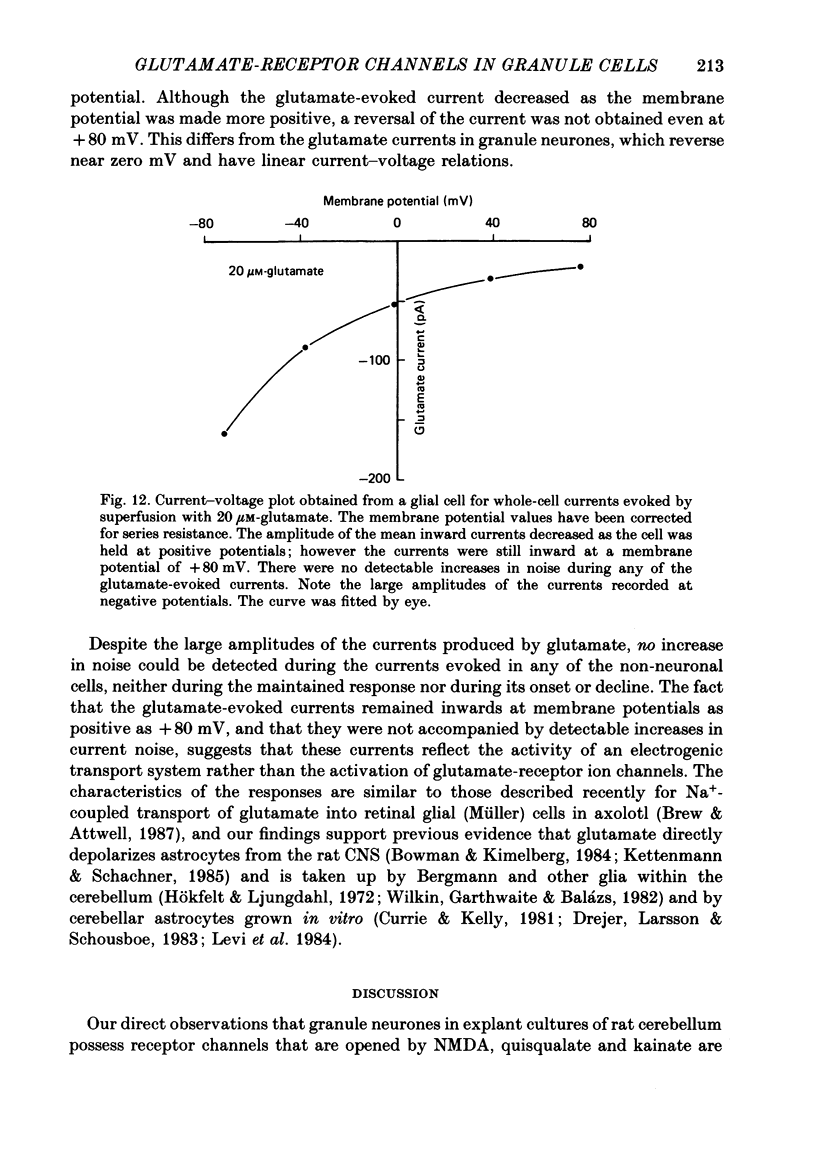
1. Glutamate-receptor ion channels in rat cerebellar granule cells maintained in explant cultures have been investigated with patch-clamp methods. Properties of these channels were determined from noise analysis of whole-cell currents and from noise and single-channel currents recorded in outside-out membrane patches. 2. Glutamate (10-20 microM) evoked two types of response. Some granule cells gave small inward currents accompanied by clear increases in current noise ('large noise' responses), whereas other cells gave larger inward currents and small noise increases ('small noise' responses). 3. A mean single-channel conductance (gamma) of 46.6 pS was estimated for glutamate from four 'large noise' cells. A mean gamma value of 8.4 pS was estimated for seven other 'large noise' cells. The results suggest that in these latter cells glutamate activated both large (approximately equal to 50 pS) and small conductance (approximately equal to 140 fS) channels. 4. Applications of aspartate (10-30 microM) or N-methyl-D-aspartate (NMDA, 10-30 microM) produced small inward currents and large increases in noise; gamma noise = 48.5 pS (aspartate) and 46.7 pS (NMDA). 5. Large single-channel currents were evoked by glutamate, aspartate and NMDA in outside-out patches. The mean conductance values obtained for the largest amplitude openings were: gamma(glutamate) = 49.5 pS, gamma(aspartate) = 51.5 pS, and gamma(NMDA) = 53.0 pS. For each agonist, these 50 pS openings comprised 75-85% of the completely resolved currents in each patch. Openings to 40 and 30 pS conductance levels accounted for 10-15% and 3-7% of the total, and the presence of apparently direct transitions between these levels and the 50 pS level suggests they are sublevels of the same multi-conductance channels. 6. A mean channel conductance of 22.9 pS was estimated from noise evoked by quisqualate (10-30 microM). Single-channel currents were examined in four patches. In two, quisqualate evoked predominantly small currents of two amplitudes, gamma = 8.4 pS and 16.5 pS; some 50 pS openings were also present. In the other two patches, most openings were 50 pS events. 7. Granule cells gave inward currents to kainate (10-30 microM), and a mean conductance of 3.1 pS was estimated from kainate noise. In patches in which aspartate or NMDA produced mainly 50 pS openings, more than 74% of the single-channel currents evoked by kainate were of smaller amplitude, with mean conductances of gamma = 8.1 and 15.1 pS.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate current noise: post-synaptic channel kinetics investigated under voltage clamp. J Physiol. 1978 Sep;282:219–242. doi: 10.1113/jphysiol.1978.sp012459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale R., Dutton G. R., Currie D. N. An ion flux assay of action potential sodium channels in neuron- and glia-enriched cultures of cells dissociated from rat cerebellum. Brain Res. 1980 Feb 3;183(1):241–246. doi: 10.1016/0006-8993(80)90137-7. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Specificity of the glial fibrillary acidic protein for astroglia. J Histochem Cytochem. 1977 Jun;25(6):466–469. doi: 10.1177/25.6.69656. [DOI] [PubMed] [Google Scholar]
- Blank N. K., Seil F. J. Reorganization in granuloprival cerebellar cultures after transplantation of granule cells and glia. II. Ultrastructural studies. J Comp Neurol. 1983 Mar 1;214(3):267–278. doi: 10.1002/cne.902140305. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Cohen J., Balázs R., Hajós F., Currie D. N., Dutton G. R. Separation of cell types from the developing cerebellum. Brain Res. 1978 Jun 16;148(2):313–331. doi: 10.1016/0006-8993(78)90722-9. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Currie D. N., Dutton G. R. [3H]GABA uptake as a marker for cell type in primary cultures of cerebellum and olfactory bulb. Brain Res. 1980 Oct 20;199(2):473–481. doi: 10.1016/0006-8993(80)90706-4. [DOI] [PubMed] [Google Scholar]
- Currie D. N., Kelly J. S. Glial versus neuronal uptake of glutamate. J Exp Biol. 1981 Dec;95:181–193. doi: 10.1242/jeb.95.1.181. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Drejer J., Larsson O. M., Schousboe A. Characterization of uptake and release processes for D- and L-aspartate in primary cultures of astrocytes and cerebellar granule cells. Neurochem Res. 1983 Feb;8(2):231–243. doi: 10.1007/BF00963923. [DOI] [PubMed] [Google Scholar]
- Dudel J. Nonlinear voltage dependence of excitatory synaptic current in crayfish muscle. Pflugers Arch. 1974;352(3):227–241. doi: 10.1007/BF00590488. [DOI] [PubMed] [Google Scholar]
- Freeman M. E., Lane J. D., Smith J. E. Turnover rates of amino acid neurotransmitters in regions of rat cerebellum. J Neurochem. 1983 May;40(5):1441–1447. doi: 10.1111/j.1471-4159.1983.tb13588.x. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Hajós F. Amino acid neurotoxicity: relationship to neuronal depolarization in rat cerebellar slices. Neuroscience. 1986 Jun;18(2):449–460. doi: 10.1016/0306-4522(86)90165-x. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Olson J. M., Penney J. B., Jr, Young A. B. Autoradiographic characterization of N-methyl-D-aspartate-, quisqualate- and kainate-sensitive glutamate binding sites. J Pharmacol Exp Ther. 1985 Apr;233(1):254–263. [PubMed] [Google Scholar]
- Hablitz J. J., Langmoen I. A. Excitation of hippocampal pyramidal cells by glutamate in the guinea-pig and rat. J Physiol. 1982 Apr;325:317–331. doi: 10.1113/jphysiol.1982.sp014152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kubo Y., Wu M. M. Cerebellar granule cells in culture: monosynaptic connections with Purkinje cells and ionic currents. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4957–4961. doi: 10.1073/pnas.83.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Autoradiographic identification of cerebral and cerebellar cortical neurons accumulating labeled gamma-aminobutyric acid ( 3 H-GABA). Exp Brain Res. 1972;14(4):354–362. doi: 10.1007/BF00235032. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Neyton J. Quisqualate and L-glutamate inhibit retinal horizontal-cell responses to kainate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1837–1841. doi: 10.1073/pnas.82.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Schachner M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J Neurosci. 1985 Dec;5(12):3295–3301. doi: 10.1523/JNEUROSCI.05-12-03295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. U. Observations on cerebellar granule cells in tissue culture. A silver and electron microscopic study. Z Zellforsch Mikrosk Anat. 1970;107(4):454–465. doi: 10.1007/BF00335434. [DOI] [PubMed] [Google Scholar]
- Kingsbury A. E., Gallo V., Woodhams P. L., Balazs R. Survival, morphology and adhesion properties of cerebellar interneurones cultured in chemically defined and serum-supplemented medium. Brain Res. 1985 Jan;349(1-2):17–25. doi: 10.1016/0165-3806(85)90128-2. [DOI] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V. Release studies related to the neurotransmitter role of glutamate in the cerebellum: an overview. Neurochem Res. 1986 Dec;11(12):1627–1642. doi: 10.1007/BF00967741. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Messer A. The maintenance and identification of mouse cerebellar granule cells in monolayer culture. Brain Res. 1977 Jul 8;130(1):1–12. doi: 10.1016/0006-8993(77)90838-1. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Characterization of excitatory amino acid receptors expressed by embryonic chick motoneurons in vitro. J Neurosci. 1986 Nov;6(11):3275–3283. doi: 10.1523/JNEUROSCI.06-11-03275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. M., Greenamyre J. T., Penney J. B., Young A. B. Autoradiographic localization of cerebellar excitatory amino acid binding sites in the mouse. Neuroscience. 1987 Sep;22(3):913–923. doi: 10.1016/0306-4522(87)92969-1. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Halasy K., Somogyi J., Storm-Mathisen J., Ottersen O. P. Quantification of immunogold labelling reveals enrichment of glutamate in mossy and parallel fibre terminals in cat cerebellum. Neuroscience. 1986 Dec;19(4):1045–1050. doi: 10.1016/0306-4522(86)90121-1. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Garthwaite J., Balázs R. Putative acidic amino acid transmitters in the cerebellum. II. Electron microscopic localization of transport sites. Brain Res. 1982 Jul 22;244(1):69–80. doi: 10.1016/0006-8993(82)90905-2. [DOI] [PubMed] [Google Scholar]
- Wolf M. K. Anatomy of cultured mouse cerebellum. II. Organotypic migration of granule cells demonstrated by silver impregnantion of normal and mutant cultures. J Comp Neurol. 1970 Nov;140(3):281–298. doi: 10.1002/cne.901400304. [DOI] [PubMed] [Google Scholar]
- Wolf M. K., Dubois-Dalcq M. Anatomy of cultured mouse cerebellum. I. Golgi and electron microscopic demonstrations of granule cells, their afferent and efferent synapses. J Comp Neurol. 1970 Nov;140(3):261–280. doi: 10.1002/cne.901400303. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Anderton B. H. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981 Mar;1(3):263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]


