Abstract
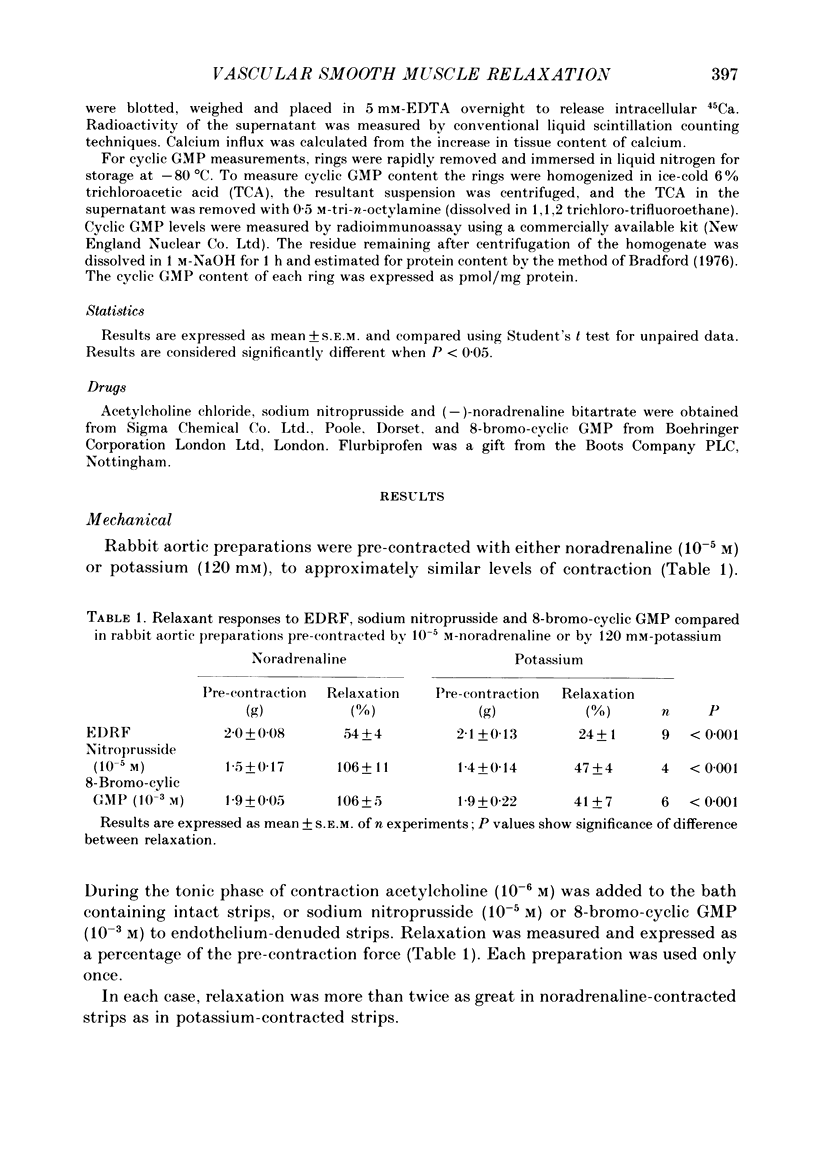
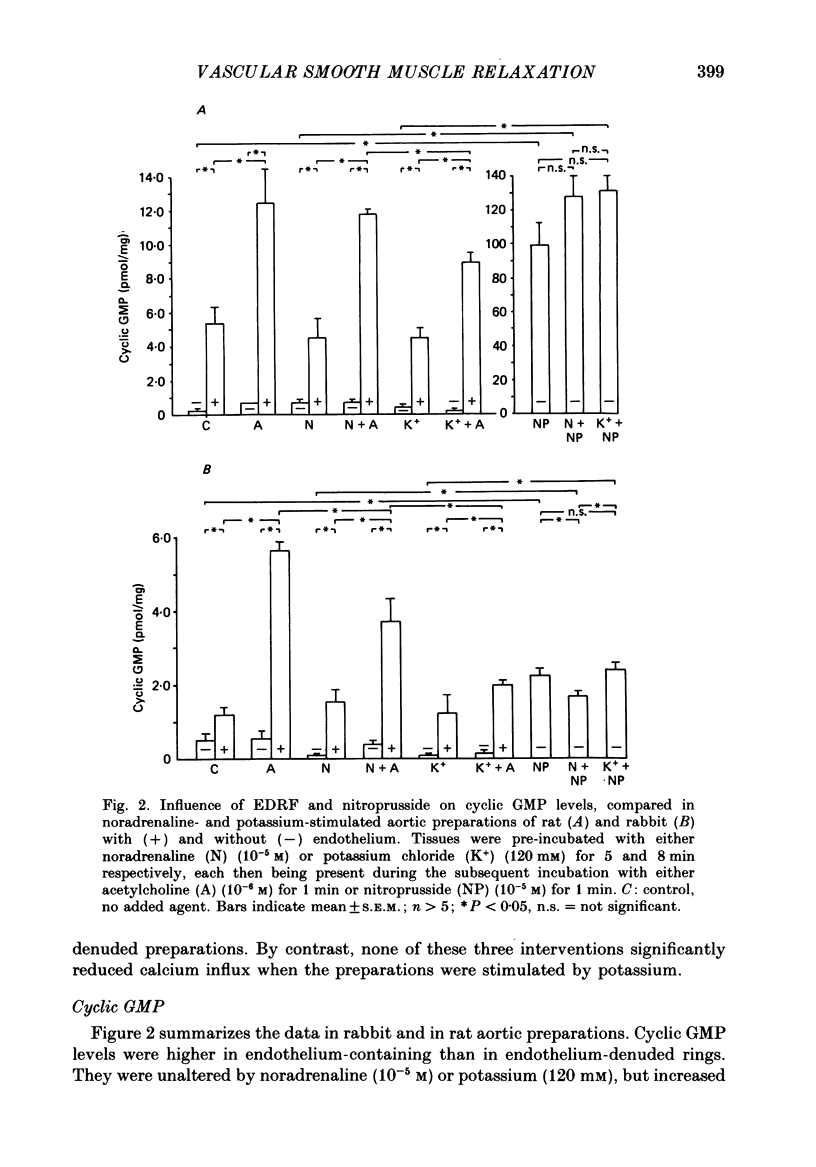
1. The effects of endothelium-derived relaxing factor (EDRF) (as stimulated by acetylcholine in the presence of endothelium), sodium nitroprusside and 8-bromocyclic GMP on mechanical relaxation, calcium (45Ca) influx and cyclic GMP levels were studied in isolated rabbit aortic preparations pre-contracted either by noradrenaline or by high (120 mM) extracellular potassium. 2. The results confirmed a relatively greater effect of these three interventions on mechanical relaxation and on reducing calcium influx in noradrenaline-contracted than in potassium-contracted preparations. 3. The increase in cyclic GMP levels induced by sodium nitroprusside, contrary to previous reports, was no greater in noradrenaline-stimulated preparations than in potassium-stimulated preparations, a finding confirmed in rat aortic preparations, and relaxation was not associated with a significant reduction of calcium influx in the potassium-stimulated preparations. 4. Cyclic GMP-mediated relaxation of potassium contraction thus appears to be due to actions of cyclic GMP other than on calcium influx. 5. These findings suggest that cyclic GMP reduces calcium influx more through receptor-operated channels than through voltage-operated channels. 6. The endothelium-dependent acetylcholine-induced elevation of cyclic GMP was reduced both by noradrenaline and by high extracellular potassium, possibly by altering release or activity of EDRF. 7. The sensitivity of the soluble guanylate cyclase system to stimulation by EDRF and nitrovasodilators appears to be greater in rat than rabbit aortic preparations.
Full text
PDF

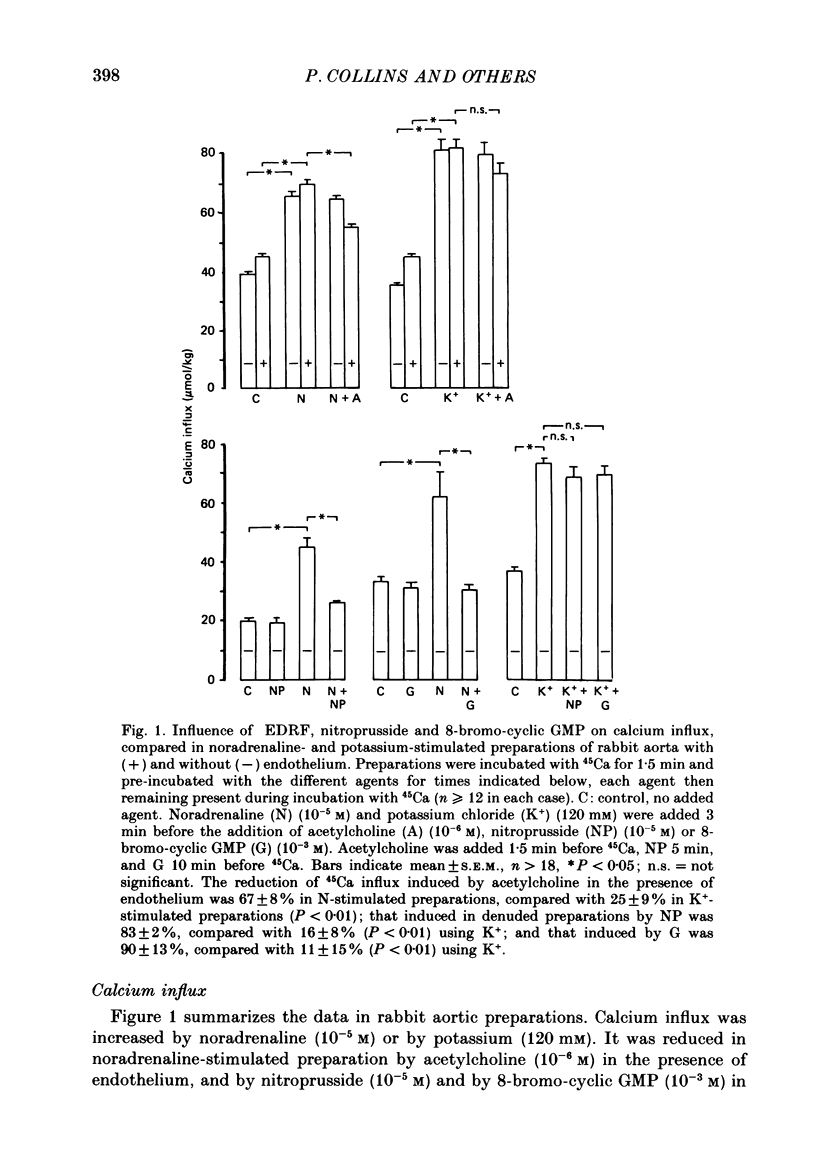





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiu P. J., Tetzloff G., Sybertz E. J. The effects of atriopeptin II on calcium fluxes in rabbit aorta. Eur J Pharmacol. 1986 May 27;124(3):277–284. doi: 10.1016/0014-2999(86)90228-1. [DOI] [PubMed] [Google Scholar]
- Collins P., Griffith T. M., Henderson A. H., Lewis M. J. Endothelium-derived relaxing factor alters calcium fluxes in rabbit aorta: a cyclic guanosine monophosphate-mediated effect. J Physiol. 1986 Dec;381:427–437. doi: 10.1113/jphysiol.1986.sp016336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986 Apr;58(4):531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- Godfraind T. EDRF and cyclic GMP control gating of receptor-operated calcium channels in vascular smooth muscle. Eur J Pharmacol. 1986 Jul 31;126(3):341–343. doi: 10.1016/0014-2999(86)90070-1. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Henderson A. H. Evidence that cyclic guanosine monophosphate (cGMP) mediates endothelium-dependent relaxation. Eur J Pharmacol. 1985 Jun 7;112(2):195–202. doi: 10.1016/0014-2999(85)90496-0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Age-related changes in the sensitivity to verapamil and sodium nitroprusside of vascular smooth muscle of rabbit aorta. Br J Pharmacol. 1985 May;85(1):223–228. doi: 10.1111/j.1476-5381.1985.tb08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Comparative effects of verapamil and sodium nitroprusside on contraction and 45Ca uptake in the smooth muscle of rabbit aorta, rat aorta and guinea-pig taenia coli. Br J Pharmacol. 1984 Feb;81(2):393–400. doi: 10.1111/j.1476-5381.1984.tb10091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Lincoln T. M. Effects of nitroprusside and 8-bromo-cyclic GMP on the contractile activity of the rat aorta. J Pharmacol Exp Ther. 1983 Jan;224(1):100–107. [PubMed] [Google Scholar]
- Malta E., Schini V., Miller R. C. Effect of endothelium on basal and alpha-adrenoceptor stimulated calcium fluxes in rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1986 Sep;334(1):63–70. doi: 10.1007/BF00498741. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Palmer R. F., Van Breemen C. The effects of amrinone on contractility, Ca2+ uptake and cAMP in smooth muscle. Eur J Pharmacol. 1980 Jan 25;61(2):159–165. doi: 10.1016/0014-2999(80)90158-2. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Taylor C. J., Saneii H. Synthetic atrial peptide inhibits intracellular calcium release in smooth muscle. Am J Physiol. 1986 Jan;250(1 Pt 1):C171–C174. doi: 10.1152/ajpcell.1986.250.1.C171. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Schwartz K., Murad F. Effect of sodium-potassium pump inhibitors and membrane-depolarizing agents on sodium nitroprusside-induced relaxation and cyclic guanosine monophosphate accumulation in rat aorta. Circ Res. 1985 Jul;57(1):164–170. doi: 10.1161/01.res.57.1.164. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Schwartz K., Murad F. Effects of Na+,K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur J Pharmacol. 1985 Apr 2;110(2):203–209. doi: 10.1016/0014-2999(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Gleason M. M., Flaim S. F. Simultaneous measurement of force and calcium uptake during acetylcholine-induced endothelium-dependent relaxation of rabbit thoracic aorta. Circ Res. 1987 Jan;60(1):31–38. doi: 10.1161/01.res.60.1.31. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Lorenz R. R., Vanhoutte P. M. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am J Physiol. 1985 Jul;249(1 Pt 2):H95–101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- Spedding M., Schini V., Schoeffter P., Miller R. C. Calcium channel activation does not increase release of endothelial-derived relaxant factors (EDRF) in rat aorta although tonic release of EDRF may modulate calcium channel activity in smooth muscle. J Cardiovasc Pharmacol. 1986 Nov-Dec;8(6):1130–1137. doi: 10.1097/00005344-198611000-00006. [DOI] [PubMed] [Google Scholar]
- Taylor C. J., Meisheri K. D. Inhibitory effects of a synthetic atrial peptide on contractions and 45Ca fluxes in vascular smooth muscle. J Pharmacol Exp Ther. 1986 Jun;237(3):803–808. [PubMed] [Google Scholar]
- Waldman S. A., Rapoport R. M., Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984 Dec 10;259(23):14332–14334. [PubMed] [Google Scholar]


