Abstract
1. Extracellular microelectrodes were used in free-to-move cats to study the locomotor-related discharges of Purkinje cells in the intermediate part of lobule V of the cerebellar anterior lobe and of neurones in the underlying nucleus interpositus anterior. All cells studied discharged rhythmically during locomotion. 2. The discharges during walking at a speed of 0.5 m/s on a horizontal exercise belt were compared with those during (a) walking at 0.9 m/s (when the duration of the step cycle is shortened considerably and the amplitudes of the locomotor electromyograms (EMGs) recorded from flexor and extensor muscles of the limbs are markedly increased) and (b) during walking at 0.5 m/s with the belt tilted uphill by 30 deg (when step duration is little changed but locomotor EMGs are increased by 70-100%). 3. In each of thirty Purkinje cells the timing of the discharges relative to the forelimb step cycle showed no major difference between the two speeds of walking. Most cells discharged at slightly higher overall rates at the faster walking speed but the increase was usually modest, the average being only 5.6 impulses/s (i.e. an increase of 8%). Peak rates sometimes underwent larger increases but the average was only 11.9 impulses/s (+11%). Changes in minimum rate were generally small (an average increase of 0.3 impulses/s). 4. Among twenty-one interpositus neurones there was only one in which discharge timing relative to the step cycle was different between the two speeds. Like the Purkinje cells, most neurones discharged slightly faster at the higher speed but the average increase was only 5.5 impulses/s (+8.5%). Peak firing rates also usually showed a modest increase (averaging 6.2 impulses/s; +6.5%) while minimum rates were little changed. 5. Among nineteen Purkinje cells compared between walking uphill and on the flat only one showed any major difference in discharge phasing; overall firing rates were on average only 1.3 impulses/s (2%) higher for uphill locomotion. 6. Among twenty-one interpositus neurones discharge phasing differed markedly between walking uphill and on the flat in only two cells. Overall discharge rates were on average slightly higher uphill (by 3.5 impulses/s; 6.7%) and peak rates also usually increased slightly (on average, by 6 impulses/s; 7.7%). Minimum rates were higher, on average, by 1.6 impulses/s (+5%). 7. The findings are discussed in relation to current notions of how the intermediate part of the cerebellum may contribute to movement control and it is concluded that the neurones studies probably make little contribution to determining the vigour of the movements of steady walking.
Full text
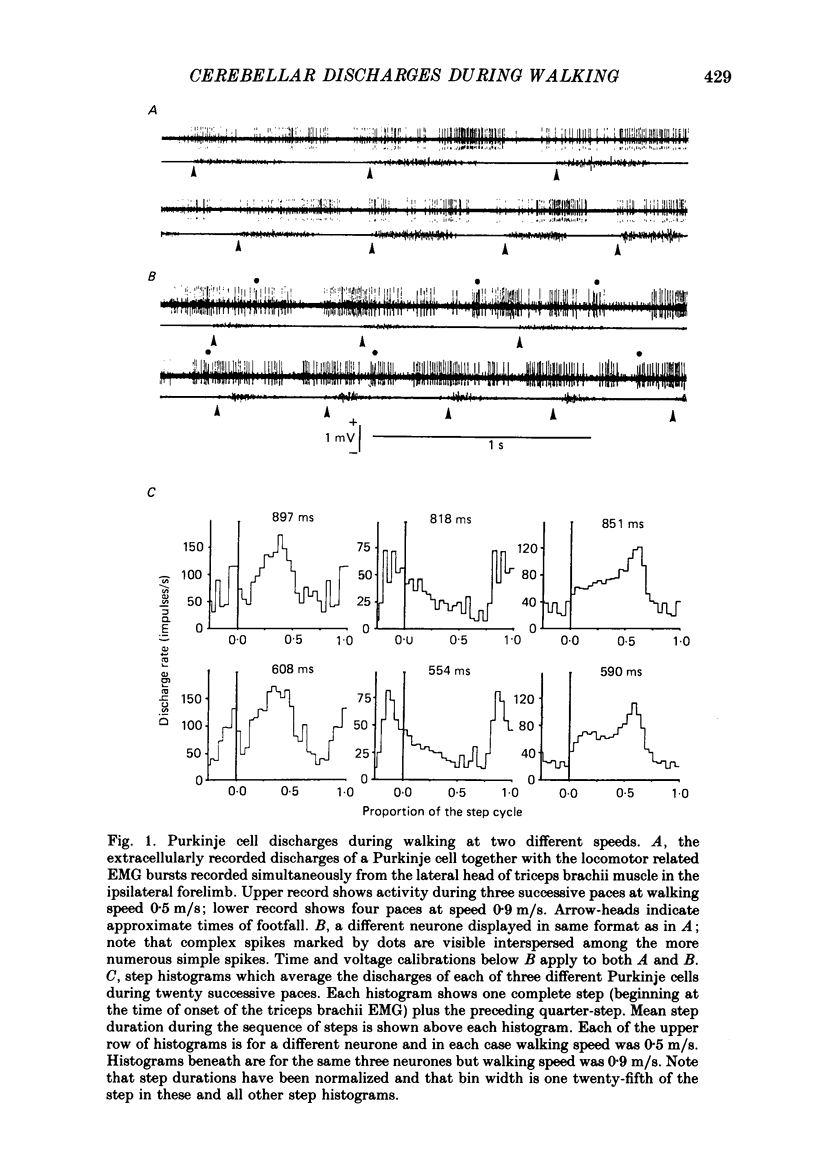
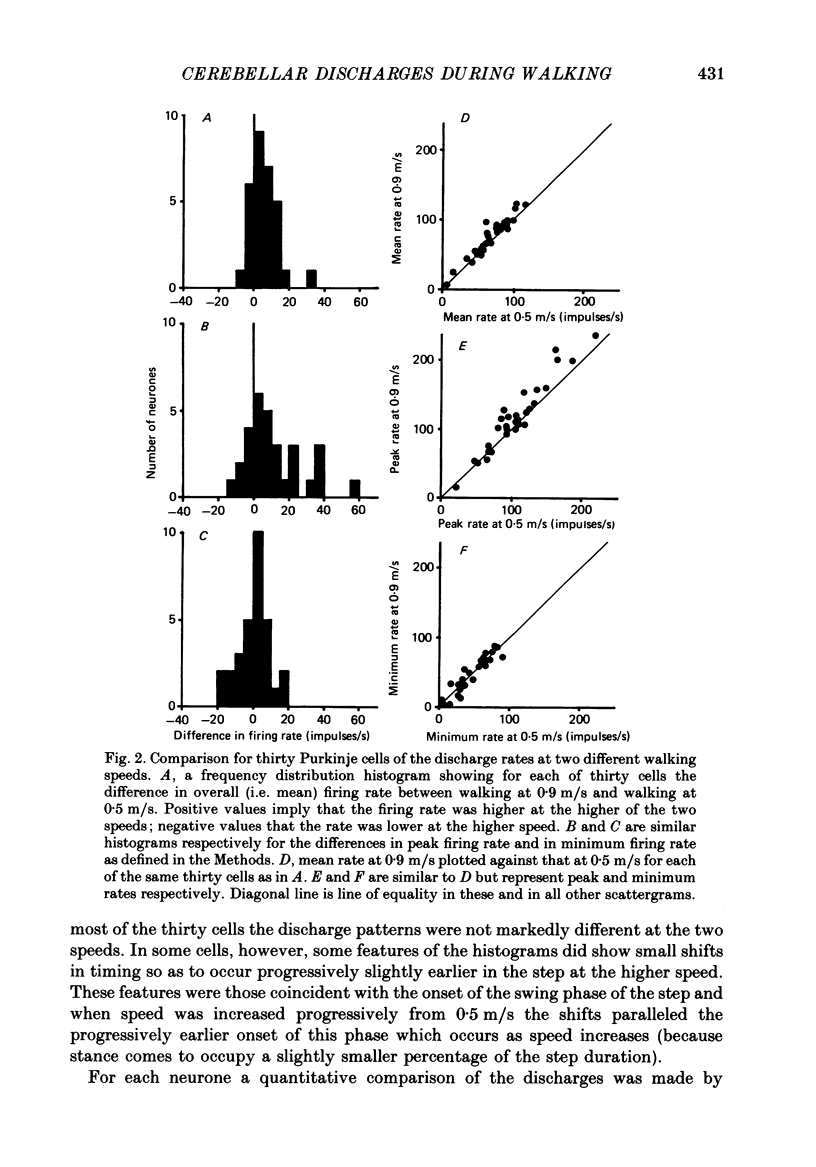
PDF





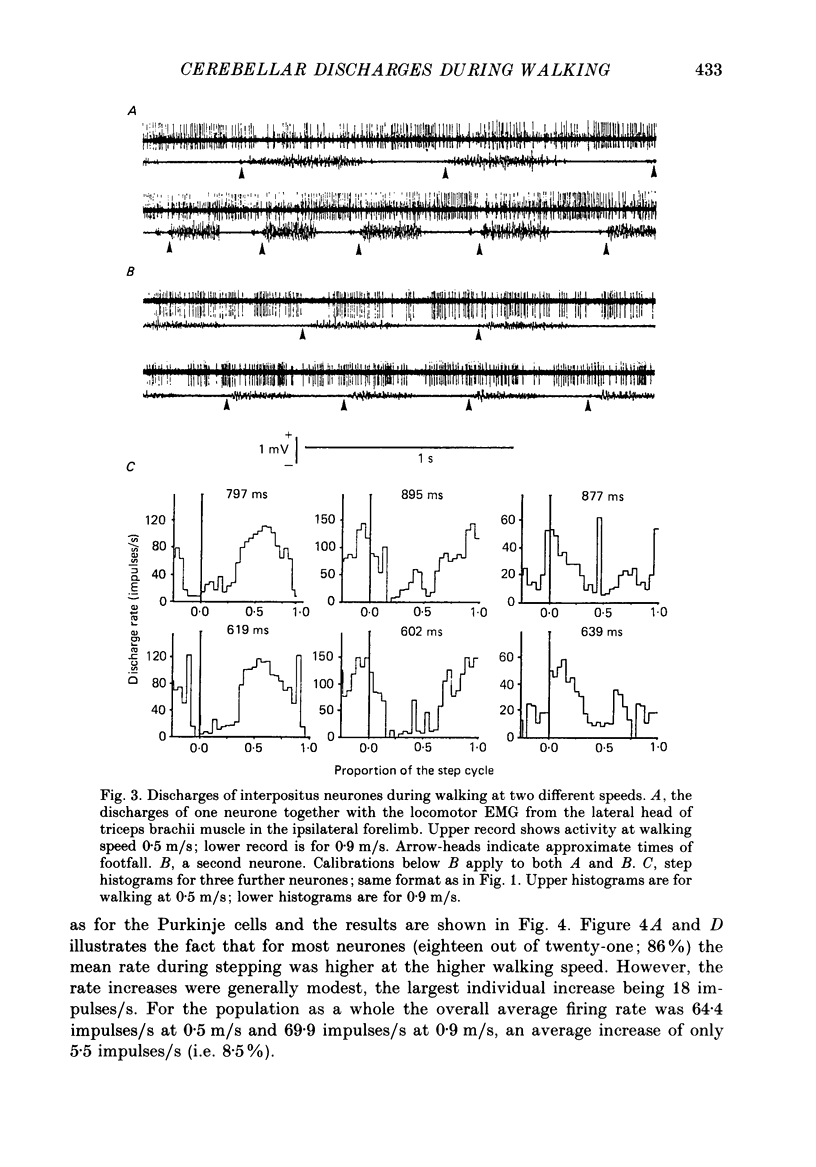
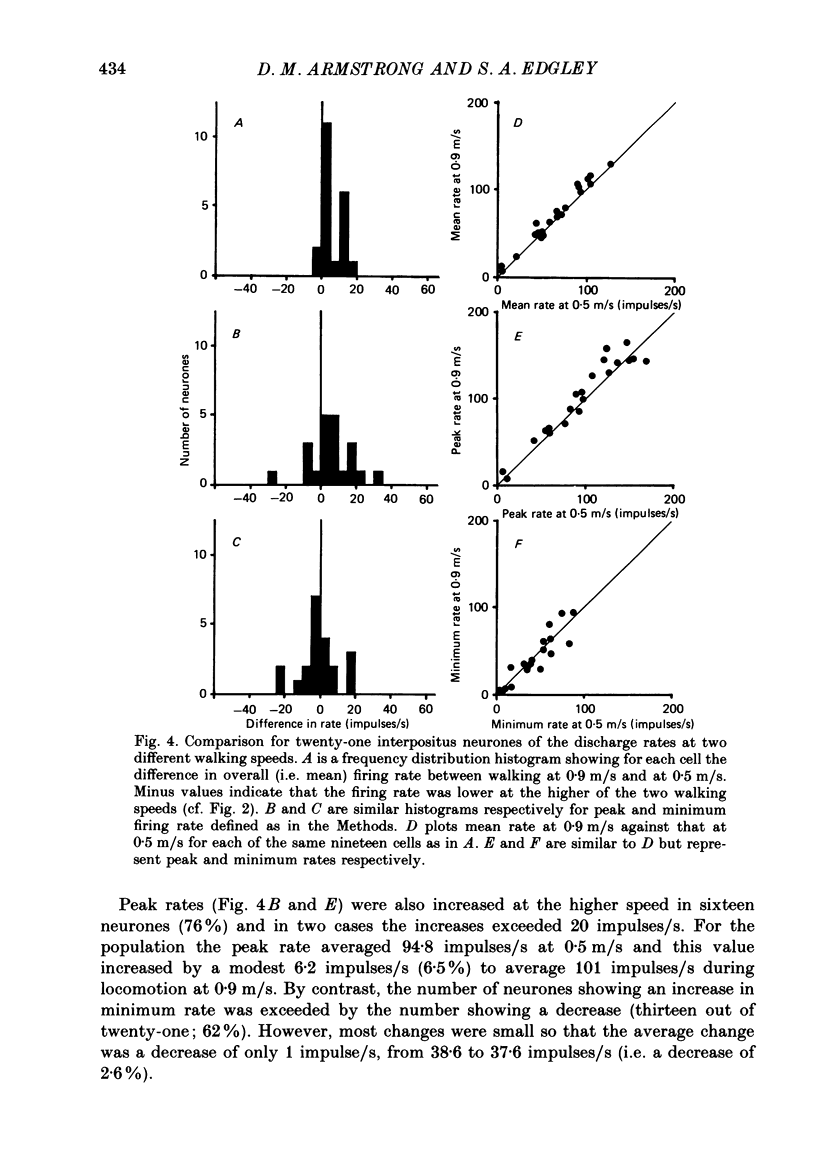







Selected References
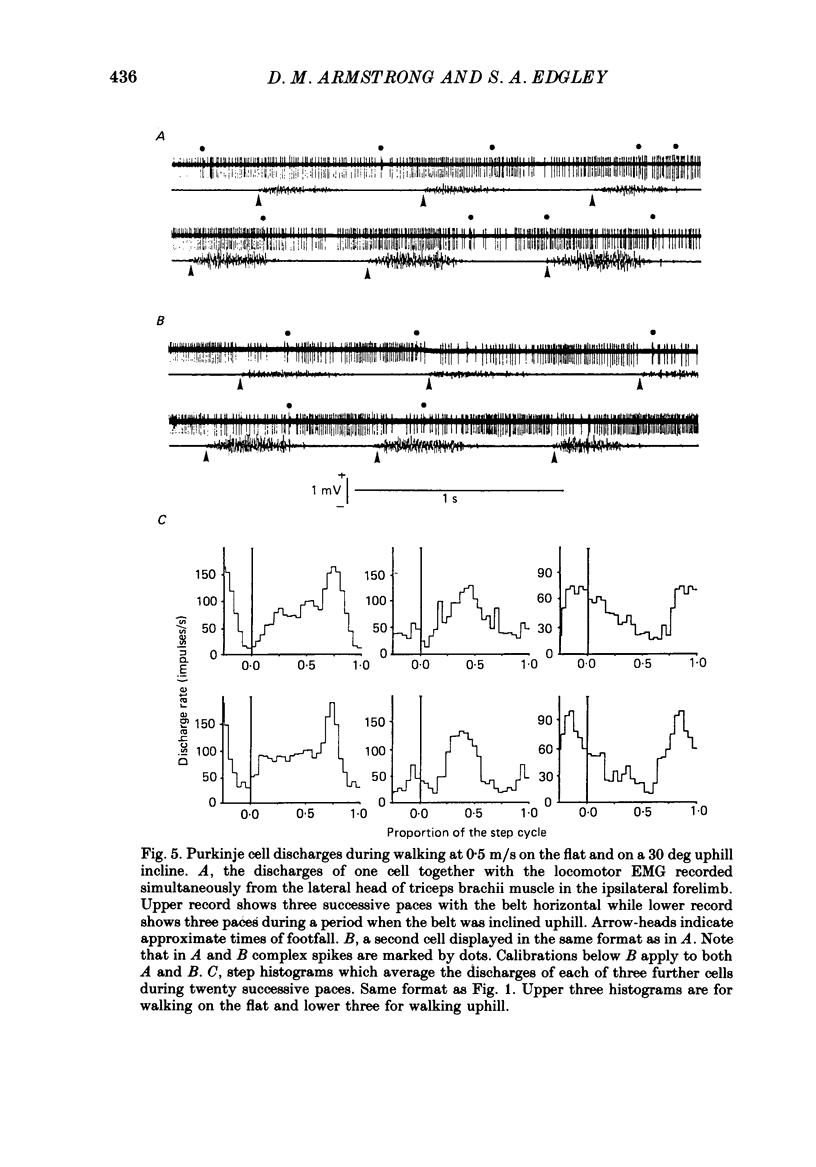
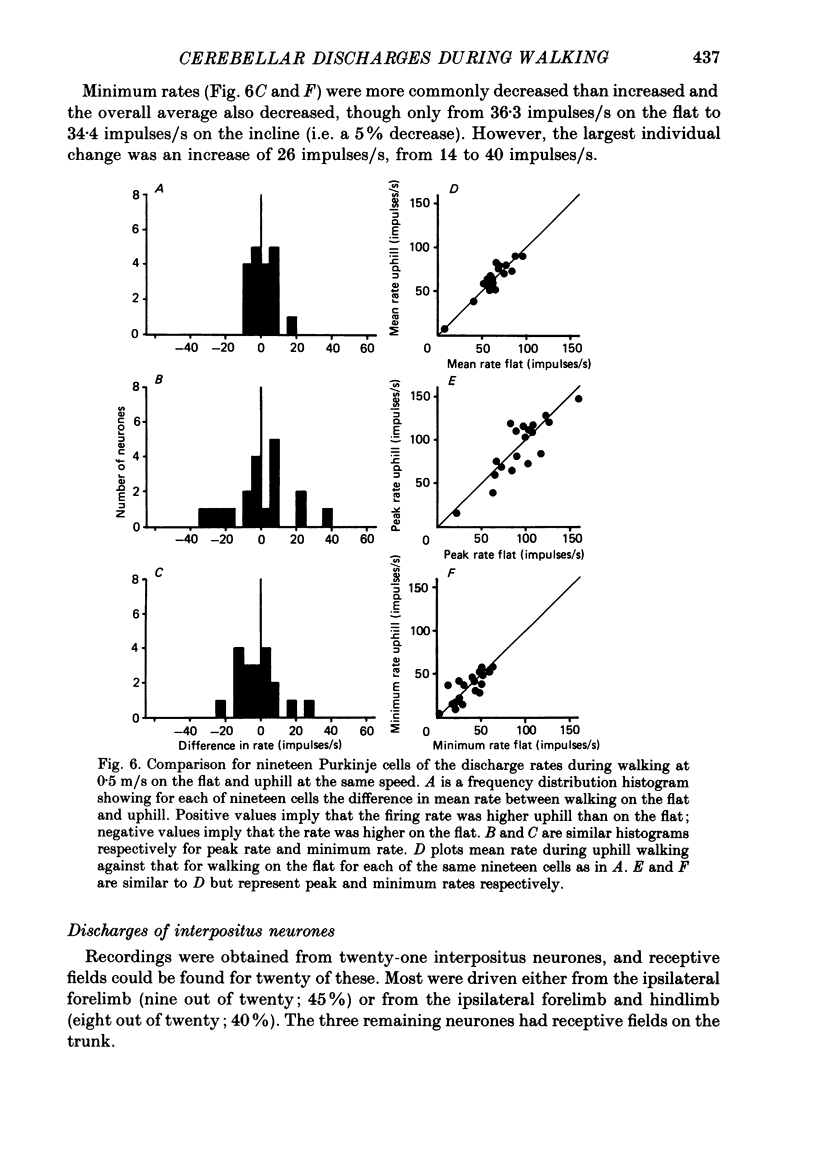
These references are in PubMed. This may not be the complete list of references from this article.
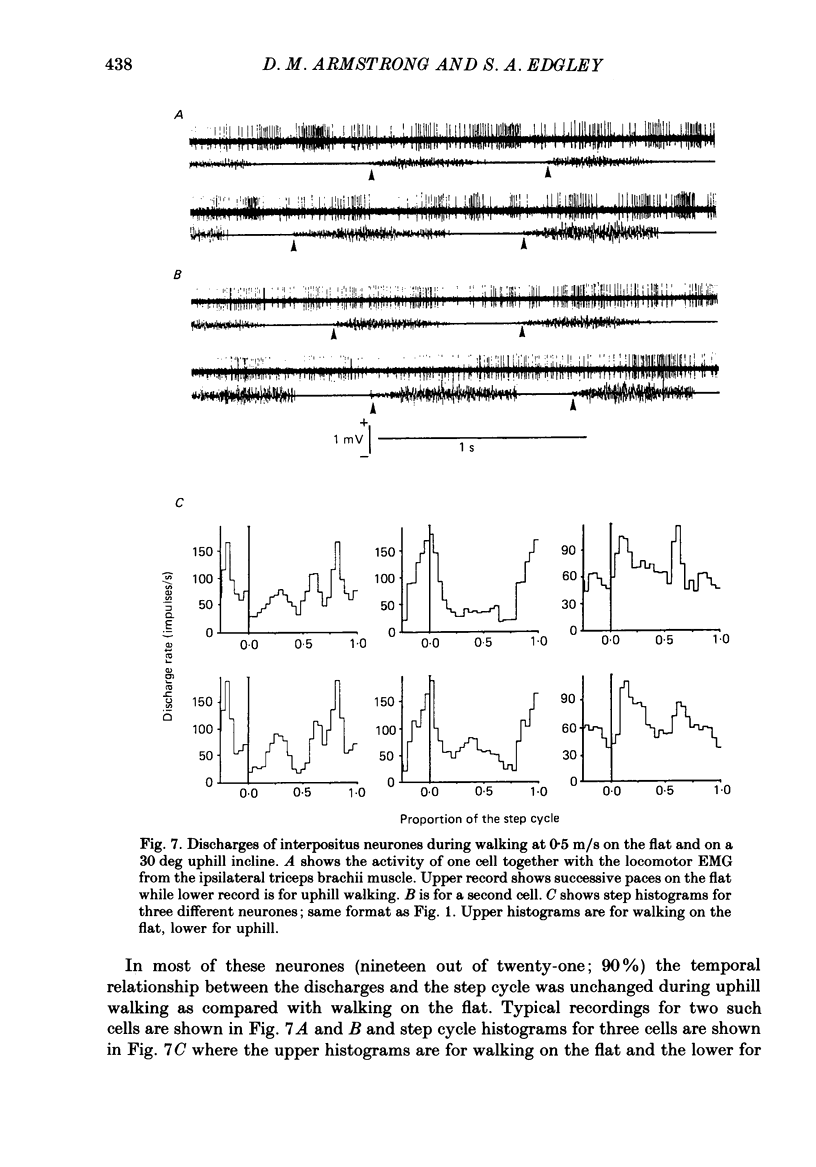
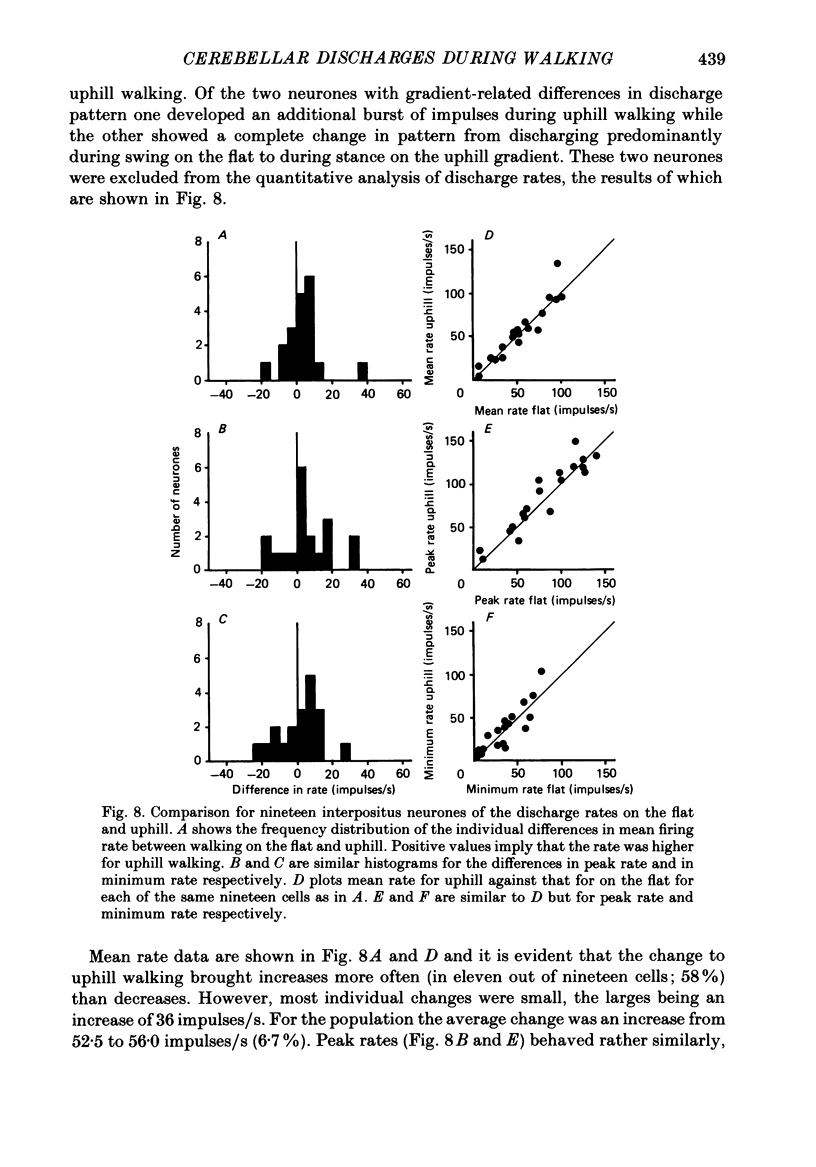
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. An intracellular study of rubrospinal and rubro-bulbospinal control of lumbar gamma-motoneurones. Acta Physiol Scand. 1982 Dec;116(4):377–386. doi: 10.1111/j.1748-1716.1982.tb07155.x. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 1984 Jul;352:403–424. doi: 10.1113/jphysiol.1984.sp015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 1984 Jun;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky Y. I., Gelfand I. M., Orlovsky G. N., Pavlova G. A. Messages conveyed by spinocerebellar pathways during scratching in the cat. I. Activity of neurons of the lateral reticular nucleus. Brain Res. 1978 Aug 11;151(3):479–491. doi: 10.1016/0006-8993(78)91081-8. [DOI] [PubMed] [Google Scholar]
- Burton J. E., Onoda N. Dependence of the activity of interpositus and red nucleus neurons on sensory input data generated by movement. Brain Res. 1978 Aug 18;152(1):41–63. doi: 10.1016/0006-8993(78)90133-6. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955 Dec;74(6):653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Lidierth M. The discharges of cerebellar Golgi cells during locomotion in the cat. J Physiol. 1987 Nov;392:315–332. doi: 10.1113/jphysiol.1987.sp016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S., Wallén P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. I. Effects on alpha-motoneurones innervating hindlimb muscles in cats. Exp Brain Res. 1969;7(4):344–364. doi: 10.1007/BF00237320. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- Mori S., Shik M. L., Yagodnitsyn A. S. Role of pontine tegmentum for locomotor control in mesencephalic cat. J Neurophysiol. 1977 Mar;40(2):284–295. doi: 10.1152/jn.1977.40.2.284. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I. Neuronal organization and synaptic mechanisms of supraspinal motor control in vertebrates. Rev Physiol Biochem Pharmacol. 1975;72:1–54. doi: 10.1007/BFb0031545. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H. Effects of partial cooling of cerebellar cortex at lobules V and IV of the intermediate part in the decerebrate walking cats under monitoring vertical floor reaction forces. Brain Res. 1979 Jan 19;160(3):559–564. doi: 10.1016/0006-8993(79)91087-4. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H. Hyperflexion and changes in interlimb coordination of locomotion induced by cooling of the cerebellar intermediate cortex in normal cats. Brain Res. 1979 Apr 27;166(2):405–408. doi: 10.1016/0006-8993(79)90228-2. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Oda Y. Cerebellar control of locomotion: effects of cooling cerebellar intermediate cortex in high decerebrate and awake walking cats. J Neurophysiol. 1980 Jul;44(1):119–134. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]
- Zajac F. E., Young J. L. Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J Neurophysiol. 1980 May;43(5):1221–1235. doi: 10.1152/jn.1980.43.5.1221. [DOI] [PubMed] [Google Scholar]



