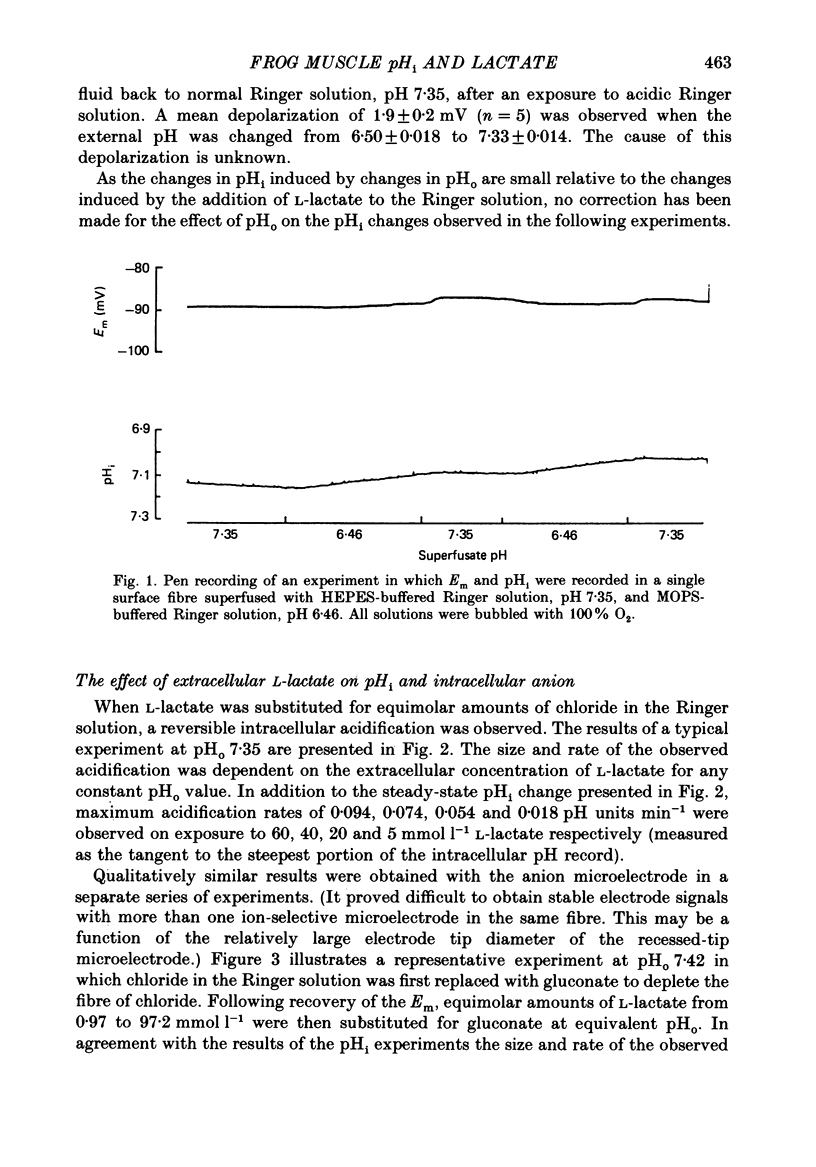
Abstract
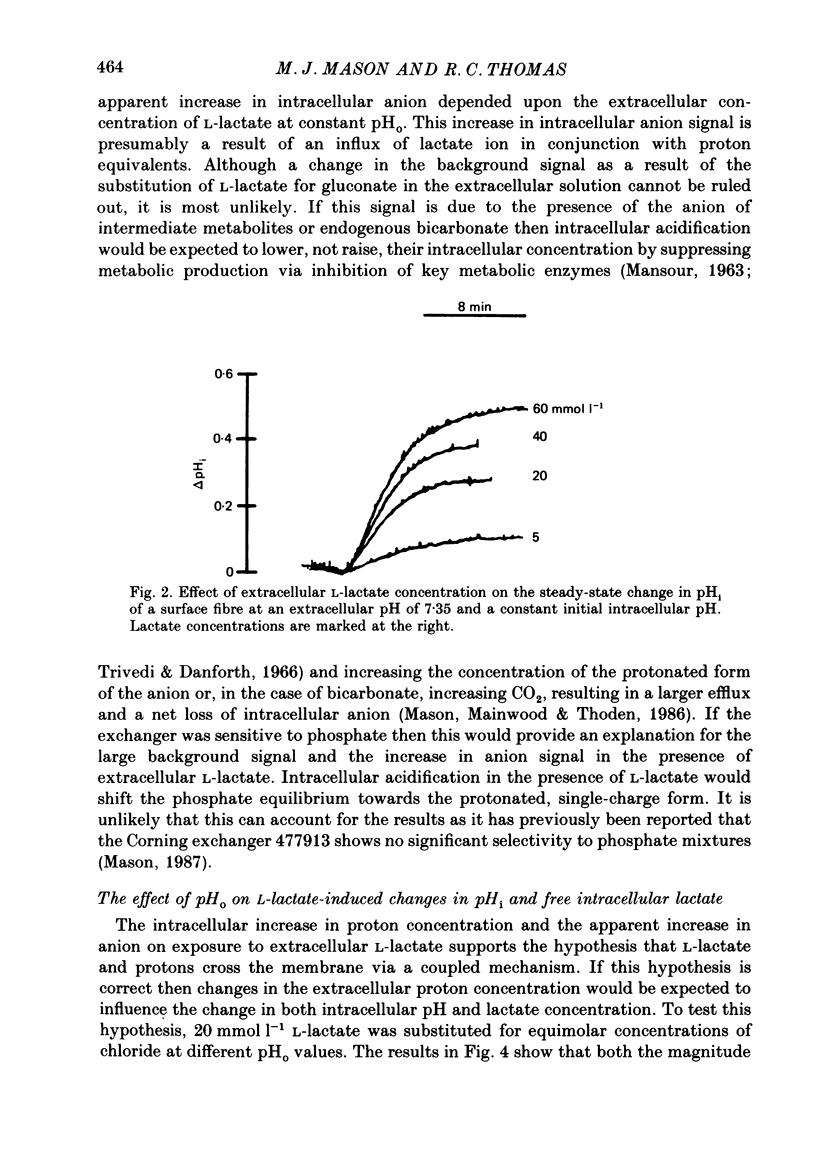
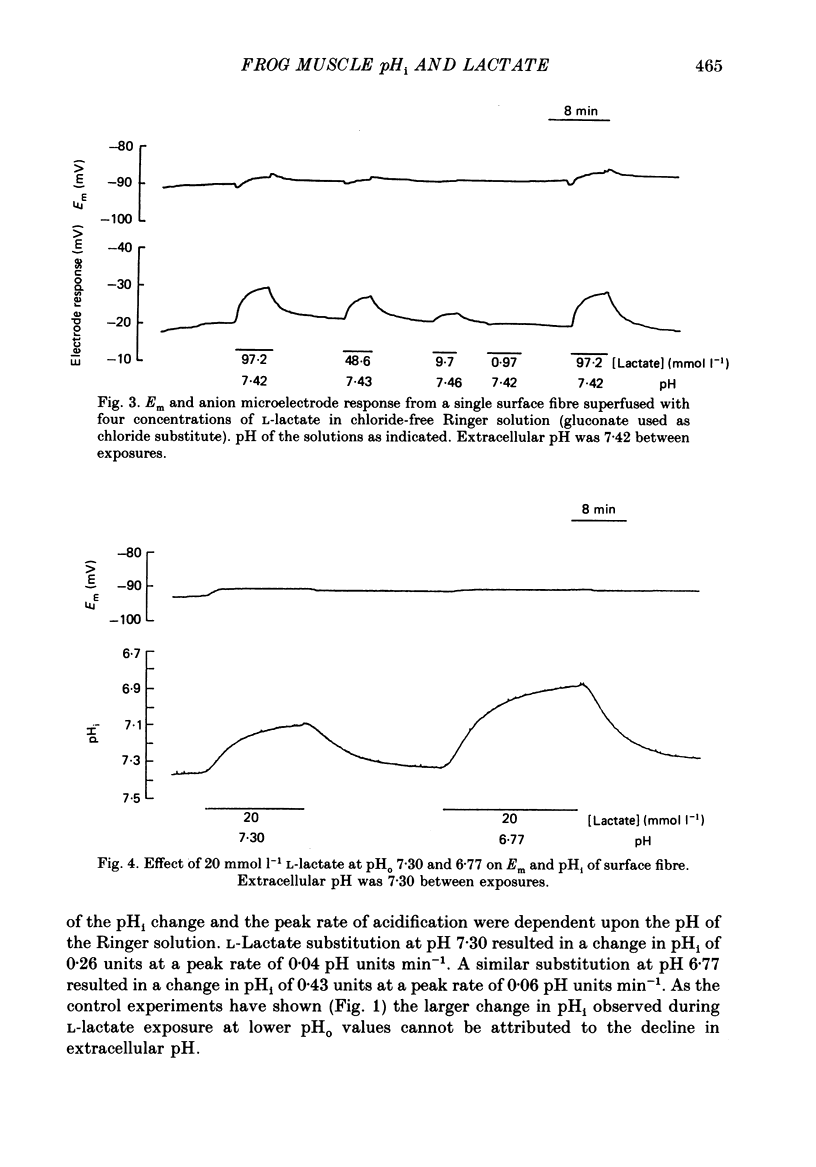
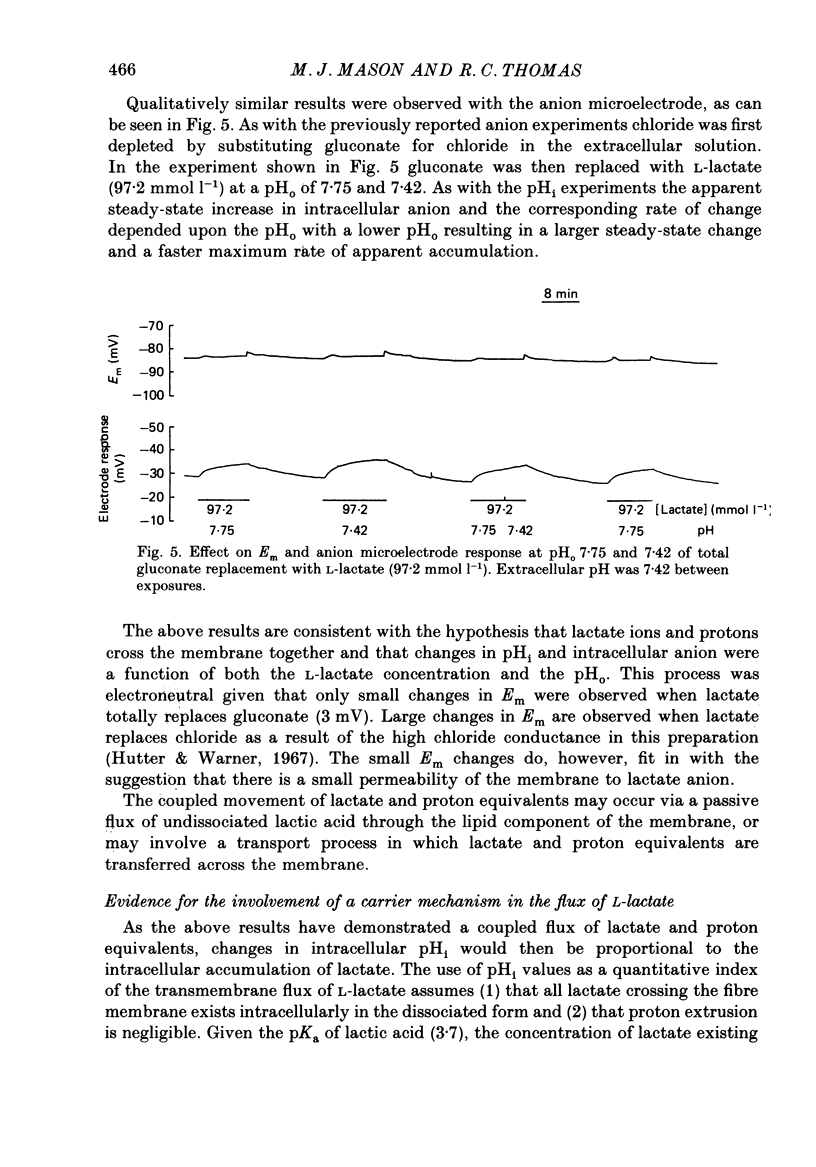
1. Changes in intracellular pH and intracellular anion levels were monitored in frog sartorius muscle fibres during exposure to extracellular L-lactate, using ion-sensitive microelectrodes. 2. Resting intracellular pH (pHi) in 20 mmol l-1 HEPES buffer was 7.18 +/- 0.015 (S.E. of mean, n = 62). Exposure to an extracellular solution at pH 6.5 buffered with 20 mmol l-1 3-(N-morpholino)propanesulphonic acid (MOPS) resulted in a slow intracellular acidification. 3. A reversible decrease in pHi and an increase in intracellular anion levels was observed when L-lactate replaced chloride in equimolar amounts. The increase in intracellular anion level is consistent with intracellular accumulation of L-lactate ion. 4. The rate and steady-state change in pHi and anion level was a function of both extracellular pH and L-lactate concentration, providing evidence for the coupled movement of lactate and proton equivalents. 5. The initial rate of uptake of L-lactate, as measured by the change of pHi, was a non-linear function of the extracellular L-lactate concentration at extracellular pH 6.8 and 7.35. 6. No saturation was observed with concentrations of L-lactate between 5 and 60 mmol l-1 at pH 7.35 and 2.5 and 40 mmol l-1 at pH 6.8. 7. The non-linear relationship between the initial rate of change in pHi and extracellular L-lactate was well fitted by a curve defining uptake as the sum of a carrier process displaying Michaelis-Menten kinetics and a passive diffusion component. The apparent Km of the carrier was 10 mmol l-1 at pHo 7.35 and 4 mmol l-1 at pHo 6.8. 8. The initial rate of change of pHi in the presence of L-lactate was significantly inhibited 39.1 +/- 6.2% by 2-5 mmol l-1 alpha-cyano-4-hydroxycinnamate (n = 9; P less than 0.05, paired t test). 9. alpha-Cyano-4-hydroxycinnamate had no detectable effect on the initial rate of change of pHi induced by propionate exposure. 10. The initial rate of change of pHi induced by L-lactate was not affected by 20-100 mumol l-1 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid (SITS). 11. We conclude that L-lactate crosses the membrane of the frog sartorius muscle with proton equivalents via (1) a carrier-mediated process, and (2) passive diffusion of lactic acid. In the physiological range of L-lactate concentrations and pH the transport process dominates.
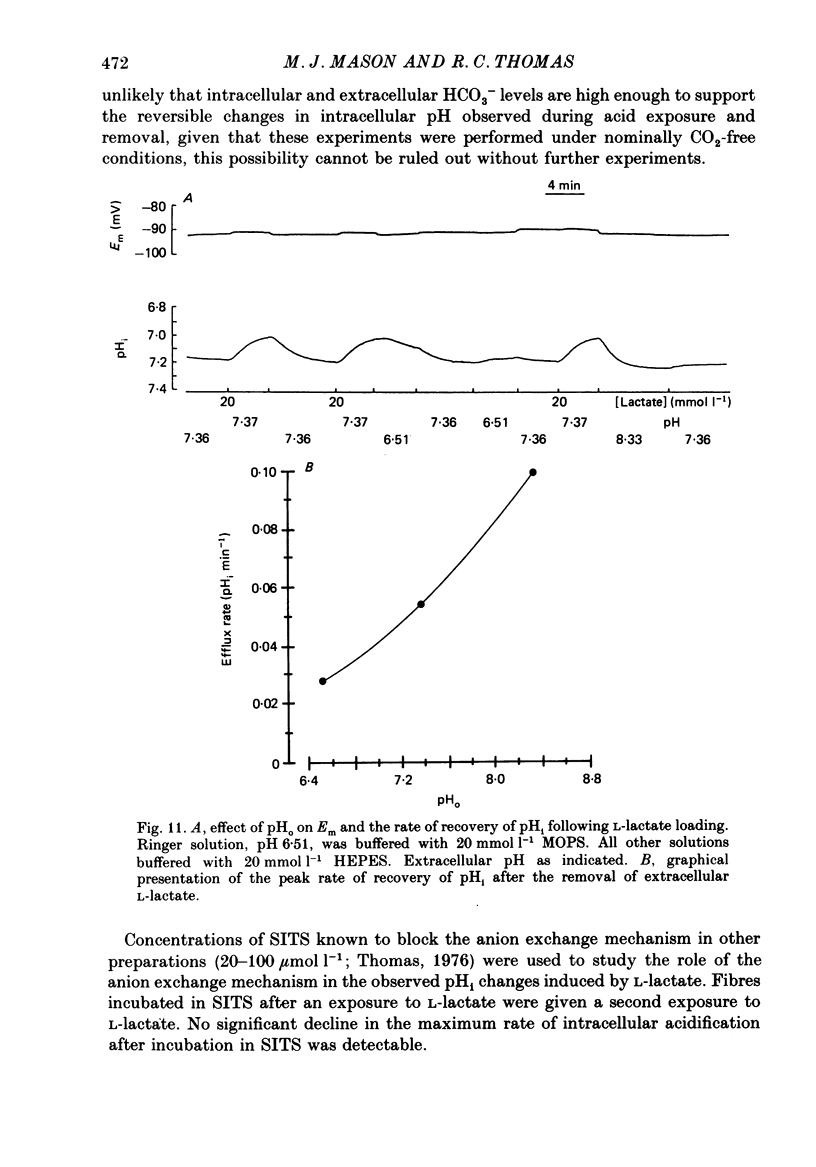
Full text
PDF



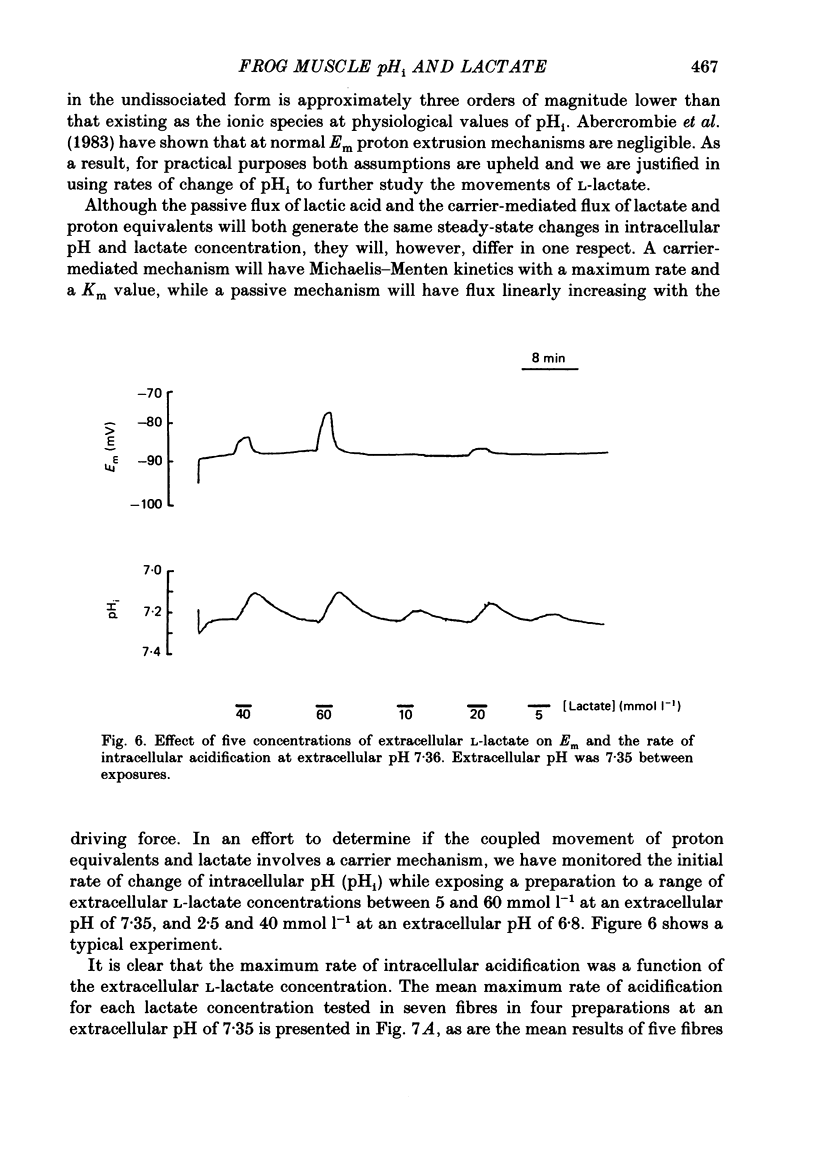








Selected References
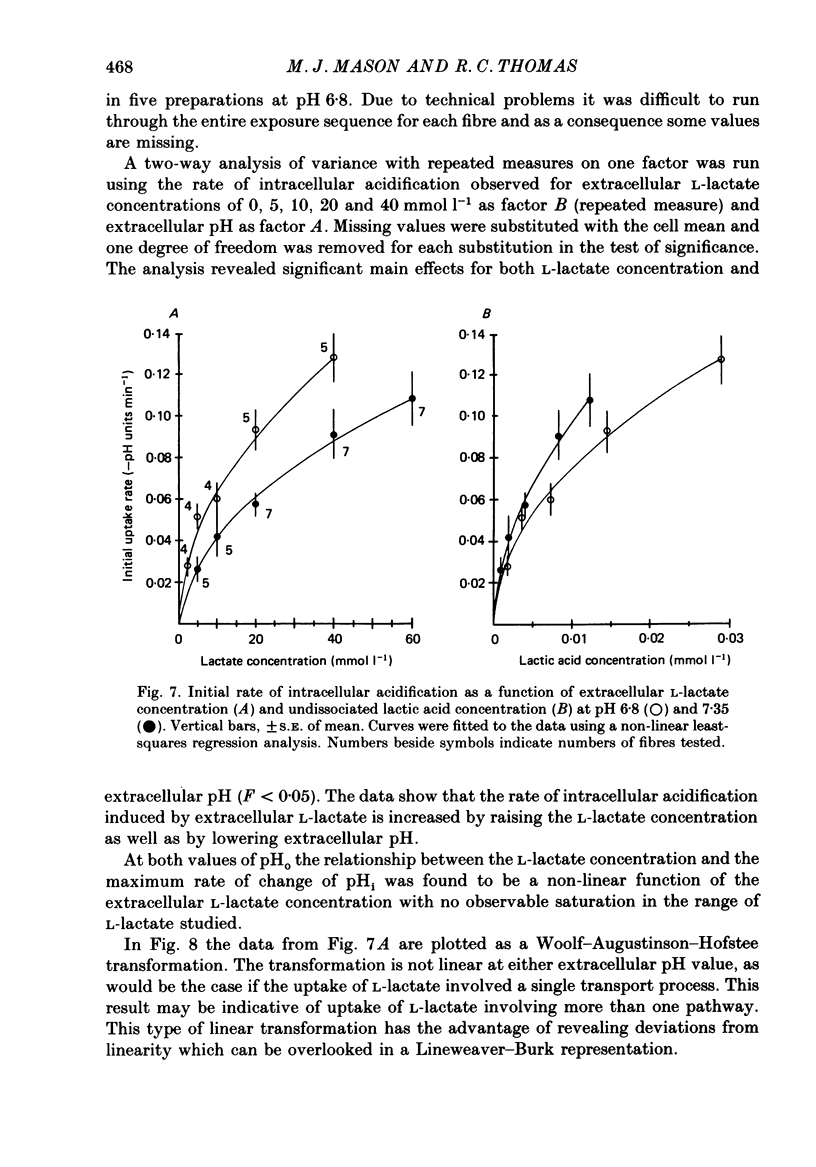
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
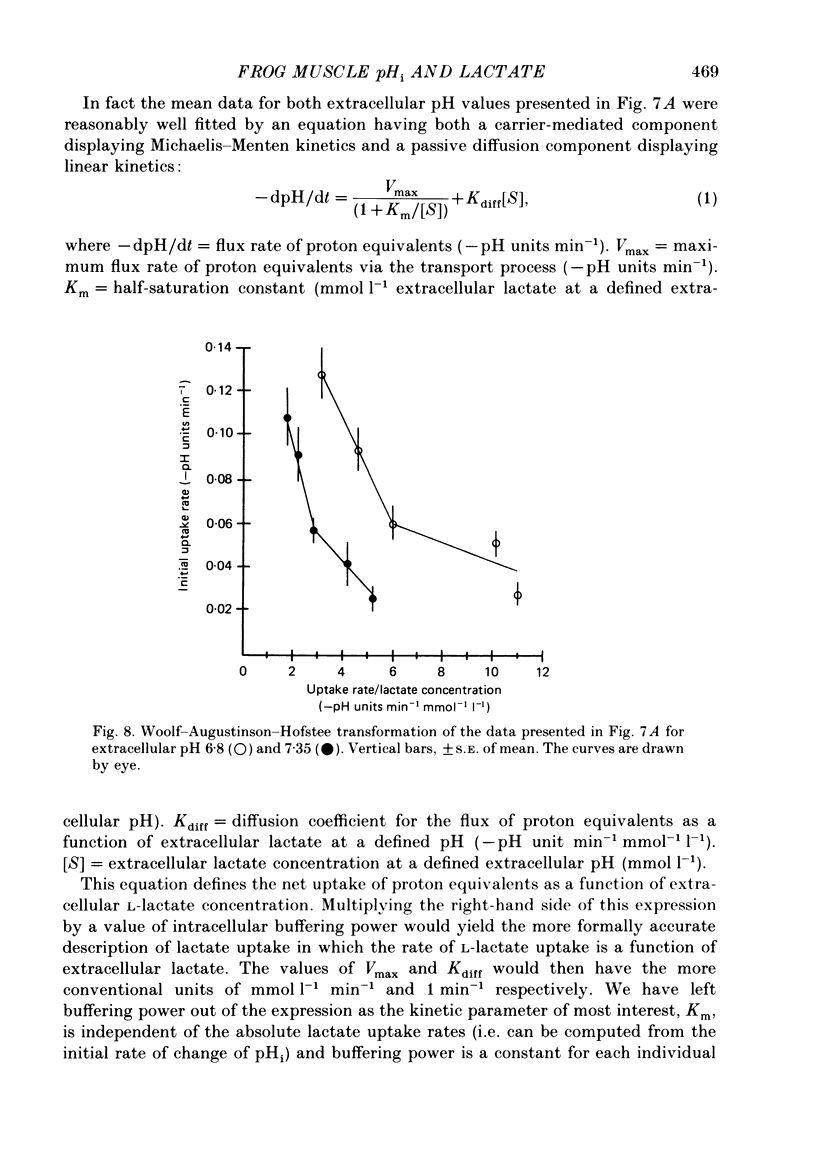
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
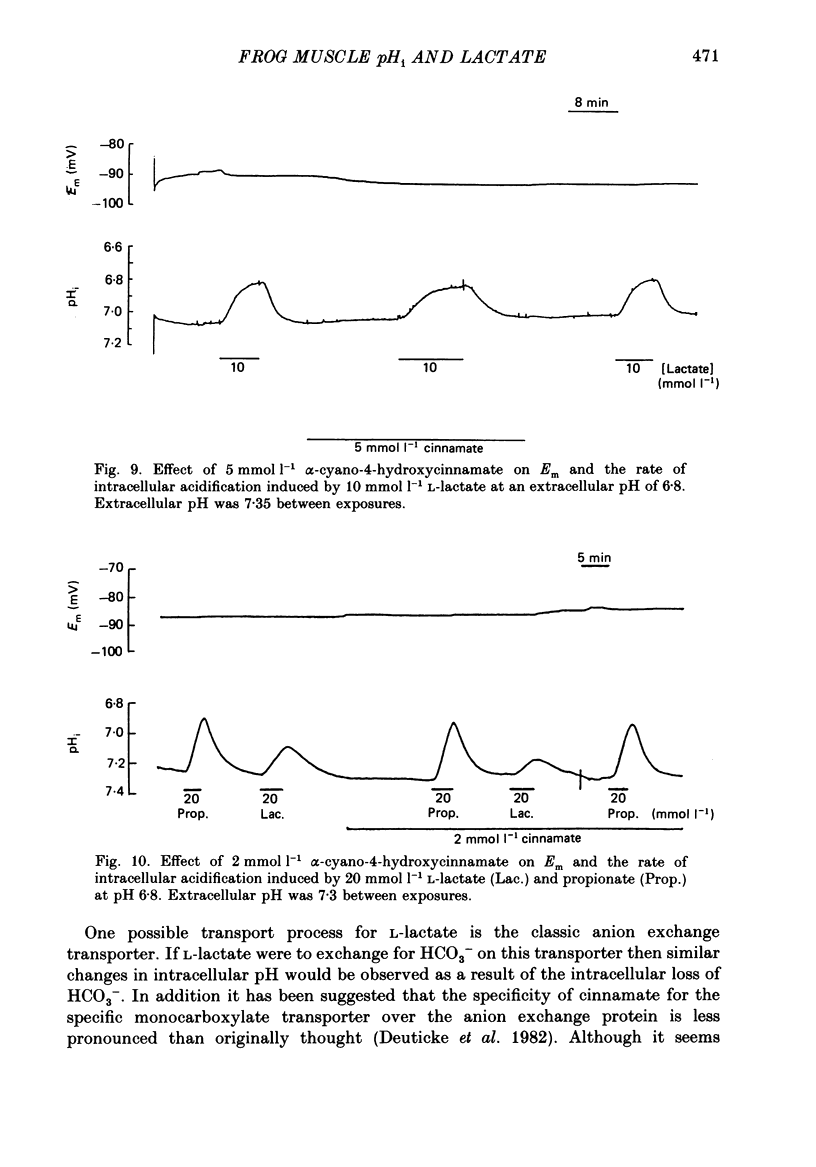
- Bendall J. R., Taylor A. A. The Meyerhof quotient and the synthesis of glycogen from lactate in frog and rabbit muscle. Biochem J. 1970 Aug;118(5):887–893. doi: 10.1042/bj1180887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Curtin N. A. Buffer power and intracellular pH of frog sartorius muscle. Biophys J. 1986 Nov;50(5):837–841. doi: 10.1016/S0006-3495(86)83524-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B., Beyer E., Forst B. Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta. 1982 Jan 4;684(1):96–110. doi: 10.1016/0005-2736(82)90053-0. [DOI] [PubMed] [Google Scholar]
- Dubinsky W. P., Racker E. The mechanism of lactate transport in human erythrocytes. J Membr Biol. 1978 Dec 8;44(1):25–36. doi: 10.1007/BF01940571. [DOI] [PubMed] [Google Scholar]
- Fafournoux P., Demigné C., Rémésy C. Carrier-mediated uptake of lactate in rat hepatocytes. Effects of pH and possible mechanisms for L-lactate transport. J Biol Chem. 1985 Jan 10;260(1):292–299. [PubMed] [Google Scholar]
- Fitts R. H., Holloszy J. O. Lactate and contractile force in frog muscle during development of fatigue and recovery. Am J Physiol. 1976 Aug;231(2):430–433. doi: 10.1152/ajplegacy.1976.231.2.430. [DOI] [PubMed] [Google Scholar]
- Fletcher W. M. Lactic acid in amphibian muscle. J Physiol. 1907 Mar 27;35(4):247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirche H. J., Hombach V., Langohr H. D., Wacker U., Busse J. Lactic acid permeation rate in working gastrocnemii of dogs during metabolic alkalosis and acidosis. Pflugers Arch. 1975;356(3):209–222. doi: 10.1007/BF00583833. [DOI] [PubMed] [Google Scholar]
- Hirche H., Hombach V., Langohr H. D., Wacker U. Lactic acid permeation rate in working skeletal muscle during alkalosis and acidosis. Pflugers Arch. 1972;332(Suppl):R73–R73. [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The effect of pH on the 36-Cl efflux from frog skeletal muscle. J Physiol. 1967 Apr;189(3):427–443. doi: 10.1113/jphysiol.1967.sp008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorfeldt L., Juhlin-Dannfelt A., Karlsson J. Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Mar;44(3):350–352. doi: 10.1152/jappl.1978.44.3.350. [DOI] [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- Koch A., Webster B., Lowell S. Cellular uptake of L-lactate in mouse diaphragm. Biophys J. 1981 Dec;36(3):775–796. doi: 10.1016/S0006-3495(81)84765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeks D. R., Halestrap A. P. Chloride-independent transport of pyruvate and lactate across the erythrocyte membrane [proceedings]. Biochem Soc Trans. 1978;6(6):1363–1366. doi: 10.1042/bst0061363. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Worsley-Brown P. The effects of extracellular pH and buffer concentration on the efflux of lactate from frog sartorius muscle. J Physiol. 1975 Aug;250(1):1–22. doi: 10.1113/jphysiol.1975.sp011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. J., Mainwood G. W., Thoden J. S. The influence of extracellular buffer concentration and propionate on lactate efflux from frog muscle. Pflugers Arch. 1986 May;406(5):472–479. doi: 10.1007/BF00583369. [DOI] [PubMed] [Google Scholar]
- Moll W., Girard H., Gros G. Facilitated diffusion of lactic acid in the guinea-pig placenta. Pflugers Arch. 1980 Jun;385(3):229–238. doi: 10.1007/BF00647462. [DOI] [PubMed] [Google Scholar]
- Monson J. P., Smith J. A., Cohen R. D., Iles R. A. Evidence for a lactate transporter in the plasma membrane of the rat hepatocyte. Clin Sci (Lond) 1982 Apr;62(4):411–420. doi: 10.1042/cs0620411. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. L., Lehninger A. L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976 Feb 15;154(2):405–414. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen C., Hirche H. J., Nestle H. W., Bovenkamp U., Hosselmann I. The interstitial pH of the working gastrocnemius muscle of the dog. Pflugers Arch. 1976 Dec 28;367(2):151–156. doi: 10.1007/BF00585151. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Comparison of the Na+ and H+ pumps in a snail neurone [proceedings]. J Physiol. 1976 Dec;263(1):212P–213P. [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- Woodbury J. W., Miles P. R. Anion conductance of frog muscle membranes: one channel, two kinds of pH dependence. J Gen Physiol. 1973 Sep;62(3):324–353. doi: 10.1085/jgp.62.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Influence of organic acids on intracellular pH. Am J Physiol. 1983 Sep;245(3):C178–C183. doi: 10.1152/ajpcell.1983.245.3.C178. [DOI] [PubMed] [Google Scholar]


