Abstract
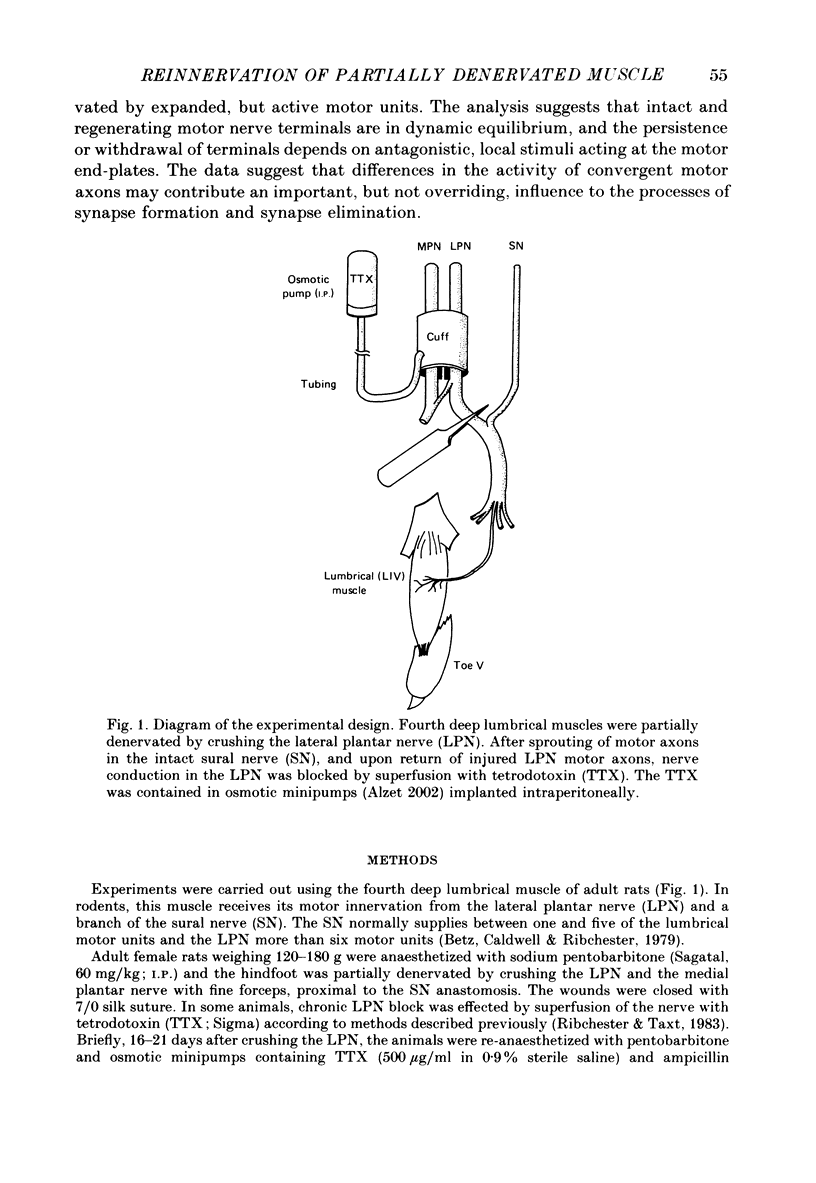
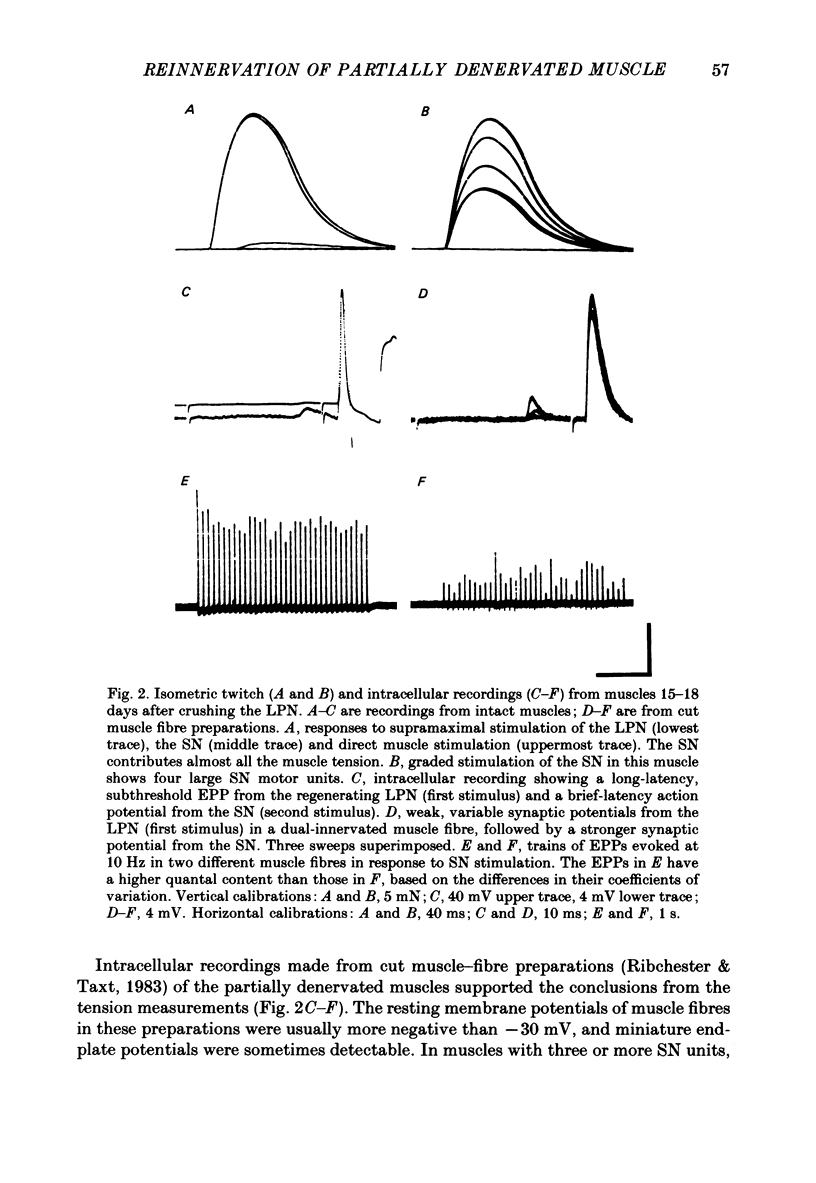
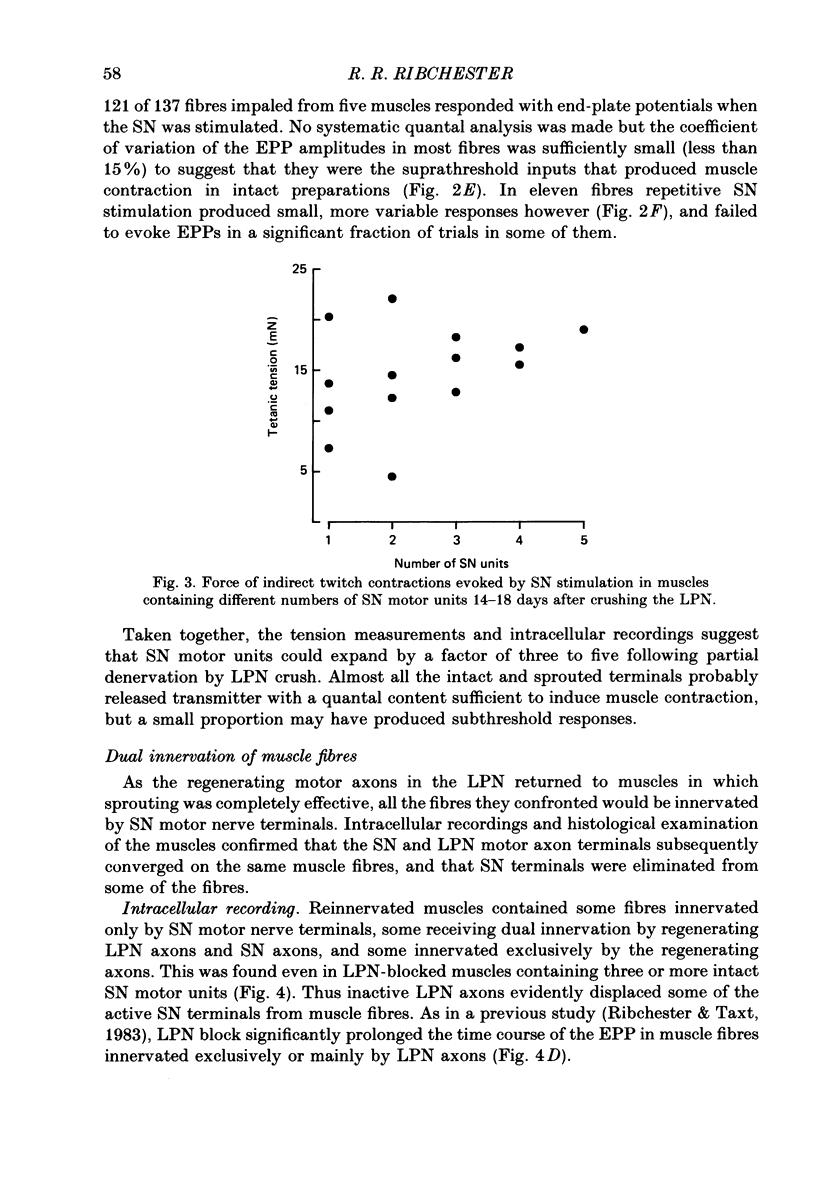
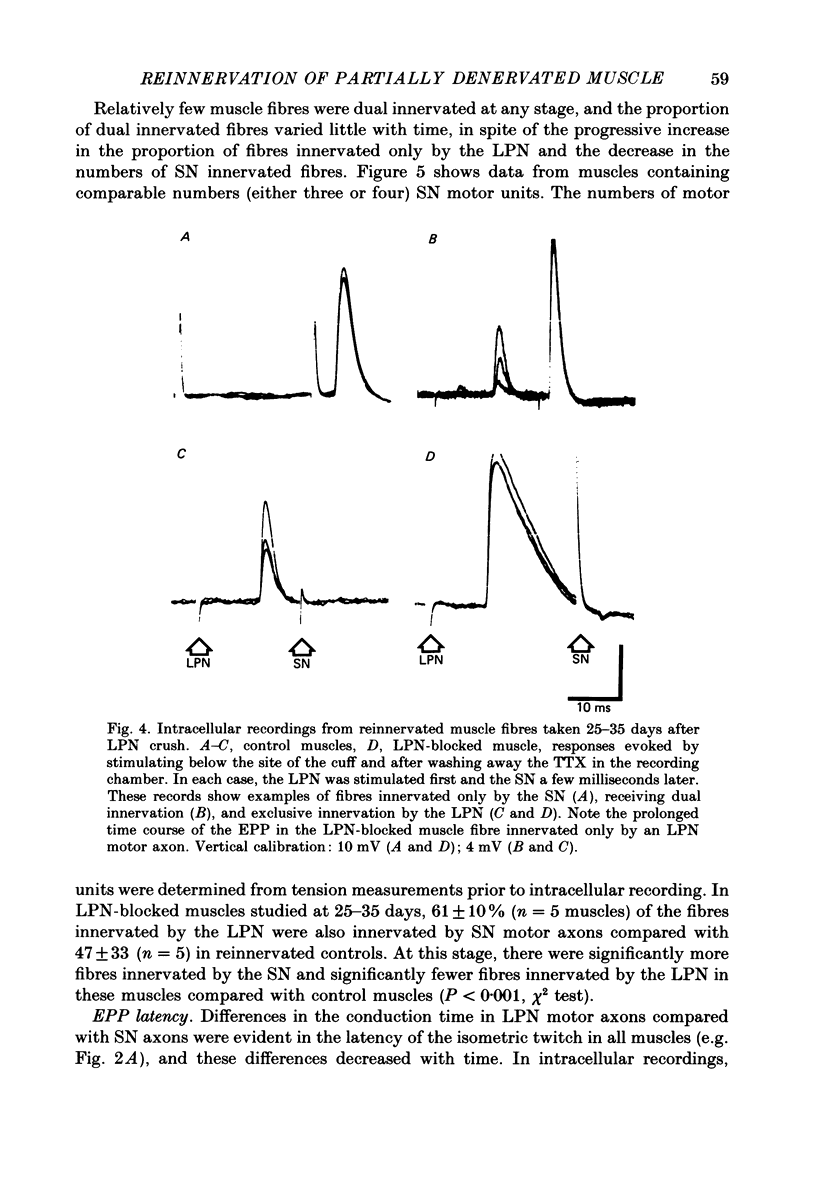
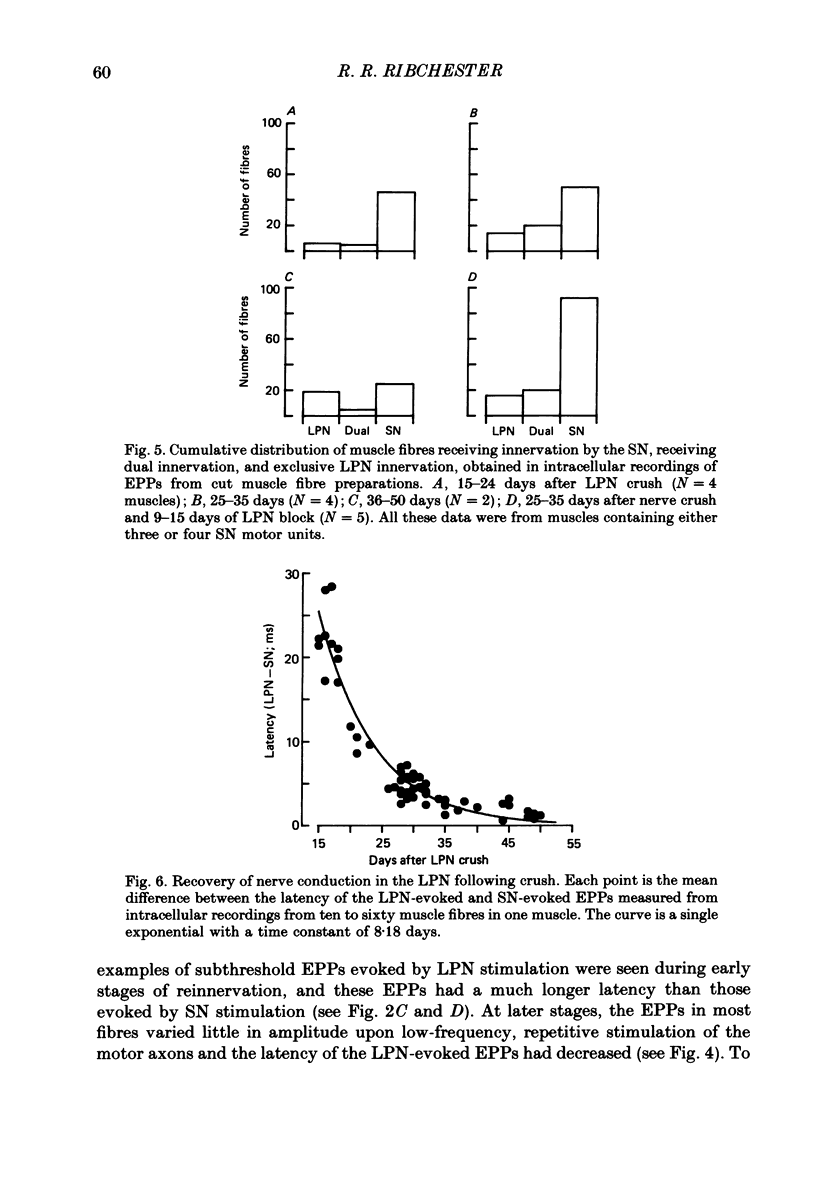
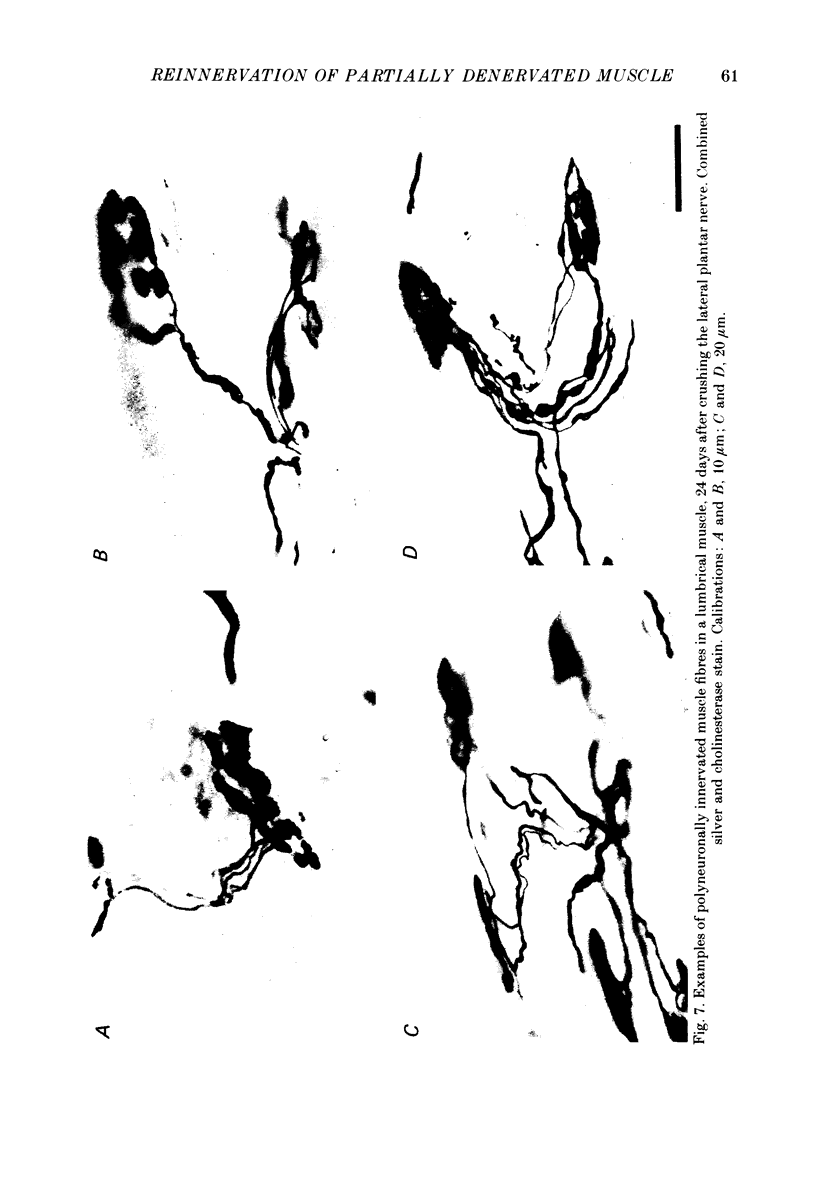
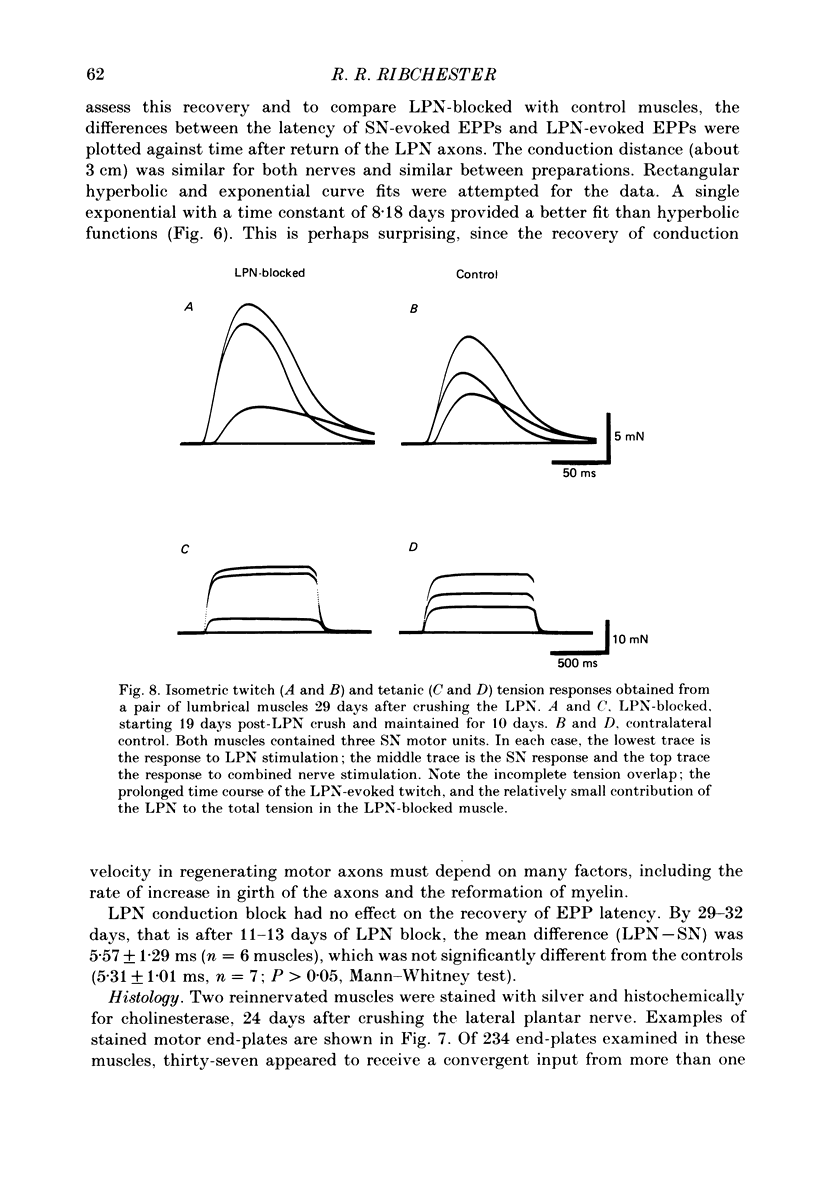
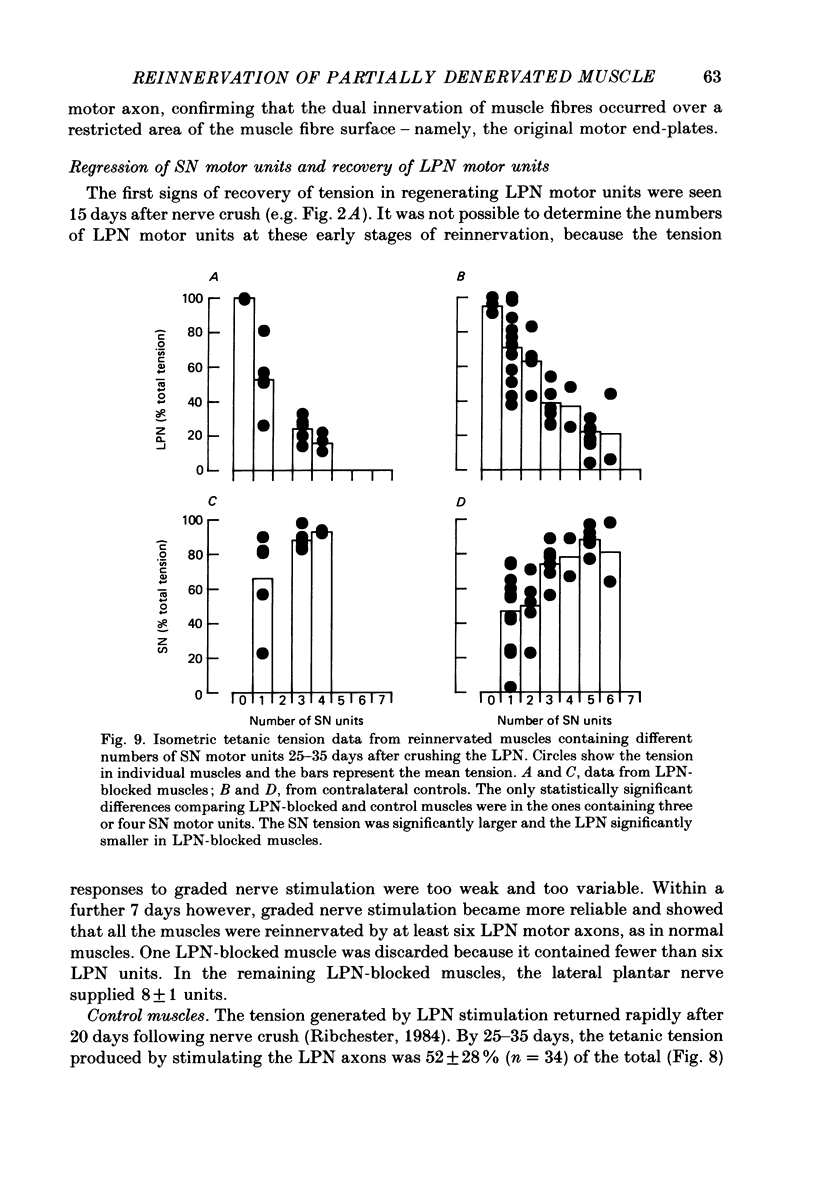
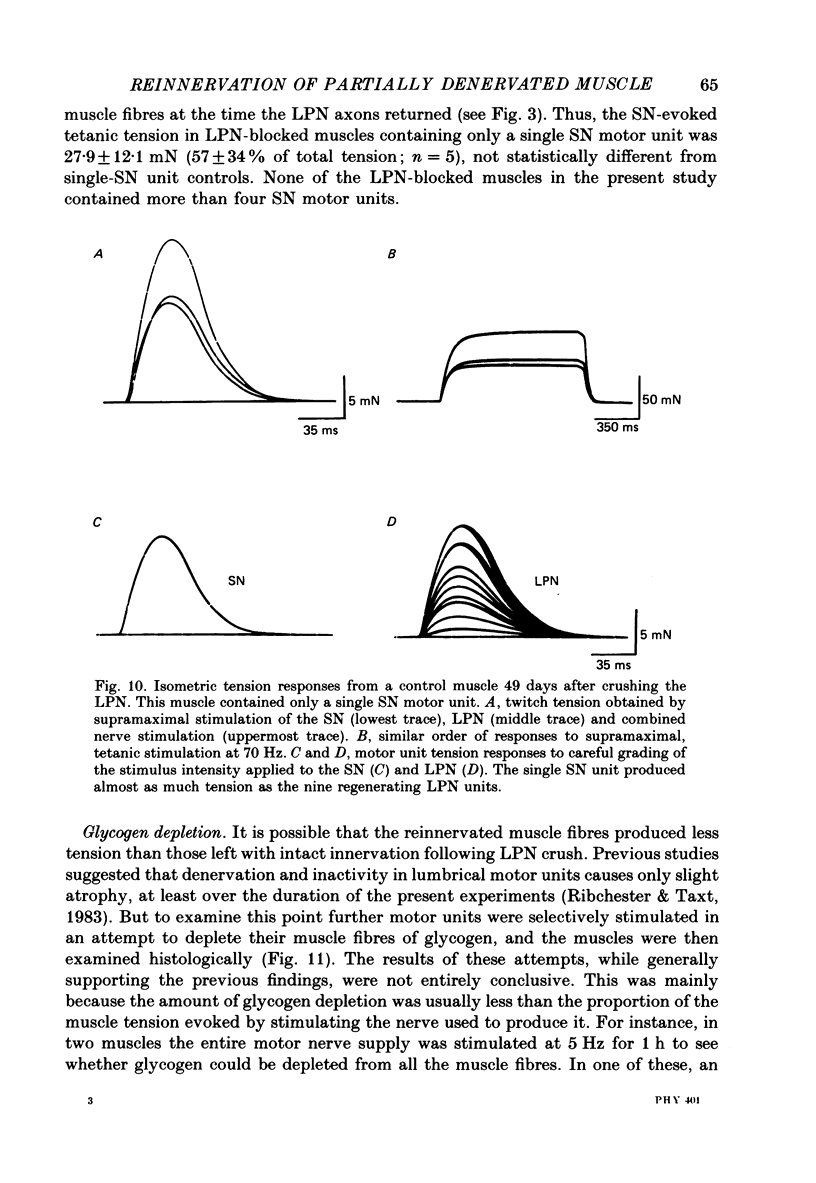
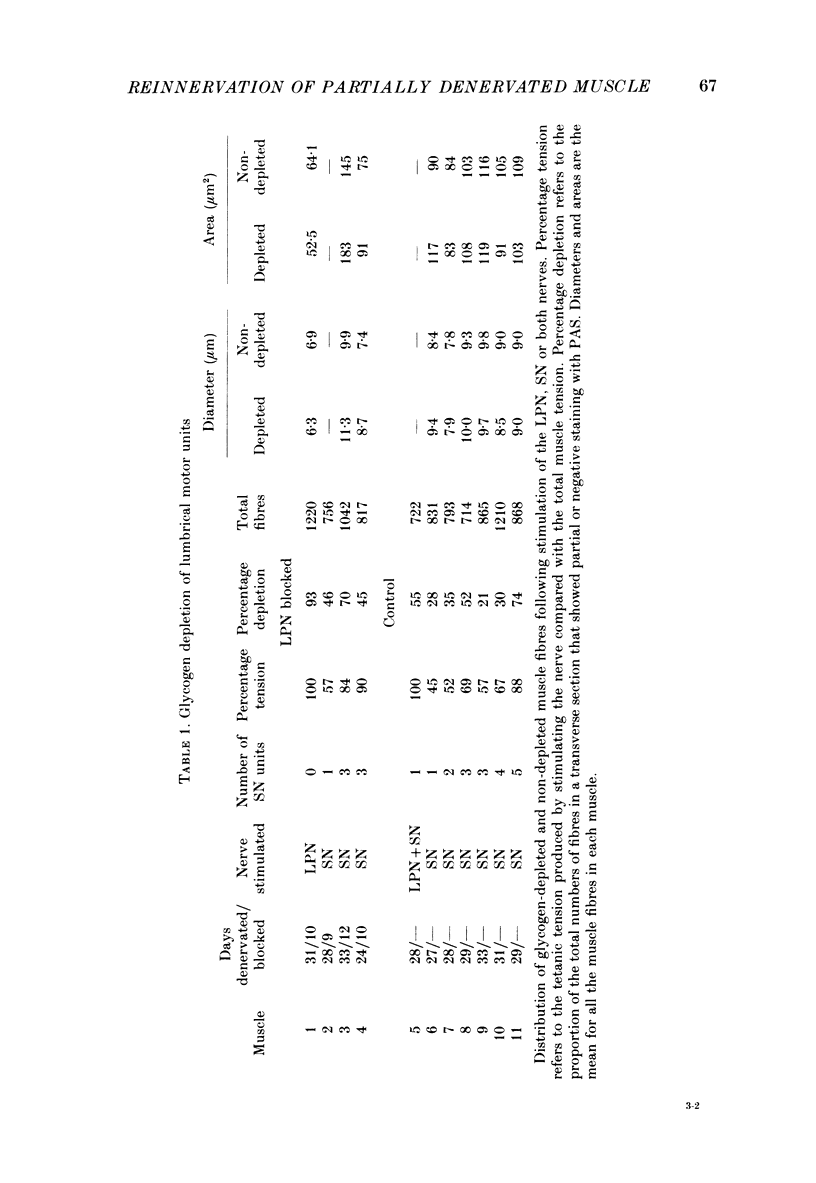
1. Reinnervation of adult rat fourth deep lumbrical muscles was studied, following extensive partial denervation of the hindfoot by crushing the lateral plantar nerve (LPN). Most muscles remained innervated by between one and five motor axons supplied by the sural nerve (SN). Intact SN motor units expanded as a result of collateral sprouting. Virtually complete collateral reinnervation occurred in muscles containing more than two SN motor units. Twitch tension measurements from isolated muscles suggested that most of the sprouts evoked suprathreshold responses from the muscle fibres they innervated. Intracellular recordings suggested that only a small percentage of sprouts evoked subthreshold end-plate potentials. 2. Lateral plantar nerve motor axons returned to the lumbrical muscles within 15-18 days and subsequently reinnervated muscle fibres already innervated by SN motor nerve terminals. Nerve conduction in the regenerating axons was then blocked for 7-15 days by chronic superfusion with tetrodotoxin. In both LPN-blocked and control (LPN crushed but not blocked) animals, isometric tetanic tension overlap and intracellular recordings showed that some lumbrical muscle fibres became innervated exclusively by regenerating LPN motor axons. 3. With time, the tension evoked by stimulating regenerating motor axons increased and there was a parallel fall in the tension produced by stimulating the intact motor units. The extent of reinnervation by LPN motor axons was inversely related to the number of remaining SN motor units. In comparable muscles, regenerated LPN-blocked motor units produced only about half the tension of the controls. Selective glycogen depletion of motor units and intracellular recordings of end-plate potentials indicated that this was due to reduced numbers of muscle fibres innervated by the blocked motor axons. 4. Nerve conduction block prolonged the time course of the isometric twitch in regenerated motor units, and increased the duration of the end-plate potential in muscle fibres innervated only by the regenerating axons. LPN block did not affect the recovery of the latency of the end-plate potential. The regenerated motor units were more resistant to fatigue caused by continuous 4 Hz nerve stimulation than intact SN units, but the resistance to fatigue of LPN-blocked motor units was no different from the controls.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz W. J., Caldwell J. H., Ribchester R. R. Sprouting of active nerve terminals in partially inactive muscles of the rat. J Physiol. 1980 Jun;303:281–297. doi: 10.1113/jphysiol.1980.sp013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Hopkins W. G., Keynes R. J. Short- and long-term effects of paralysis on the motor innervation of two different neonatal mouse muscles. J Physiol. 1982 Aug;329:439–450. doi: 10.1113/jphysiol.1982.sp014312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. M., Soha J. M., Van Essen D. C. Competition favouring inactive over active motor neurons during synapse elimination. 1987 Jul 30-Aug 5Nature. 328(6129):422–426. doi: 10.1038/328422a0. [DOI] [PubMed] [Google Scholar]
- Campenot R. B. Retraction and degeneration of sympathetic neurites in response to locally elevated potassium. Brain Res. 1986 Dec 10;399(2):357–363. doi: 10.1016/0006-8993(86)91528-3. [DOI] [PubMed] [Google Scholar]
- Chapman B., Jacobson M. D., Reiter H. O., Stryker M. P. Ocular dominance shift in kitten visual cortex caused by imbalance in retinal electrical activity. Nature. 1986 Nov 13;324(6093):154–156. doi: 10.1038/324154a0. [DOI] [PubMed] [Google Scholar]
- Connold A. L., Evers J. V., Vrbová G. Effect of low calcium and protease inhibitors on synapse elimination during postnatal development in the rat soleus muscle. Brain Res. 1986 Jul;393(1):99–107. doi: 10.1016/0165-3806(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cotter M., Phillips P. Rapid fast to slow fiber transformation in response to chronic stimulation of immobilized muscles of the rabbit. Exp Neurol. 1986 Sep;93(3):531–545. doi: 10.1016/0014-4886(86)90173-1. [DOI] [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- Dohrmann U., Edgar D., Sendtner M., Thoenen H. Muscle-derived factors that support survival and promote fiber outgrowth from embryonic chick spinal motor neurons in culture. Dev Biol. 1986 Nov;118(1):209–221. doi: 10.1016/0012-1606(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C., Nelson P. G. Depolarization-induced synaptic plasticity at cholinergic synapses in tissue culture. J Neurosci. 1981 Sep;1(9):1043–1051. doi: 10.1523/JNEUROSCI.01-09-01043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH L. Neuromuscular function after regeneration of interrupted nerve fibers into partially denervated muscle. Exp Neurol. 1962 Aug;6:129–141. doi: 10.1016/0014-4886(62)90083-3. [DOI] [PubMed] [Google Scholar]
- Gouzé J. L., Lasry J. M., Changeux J. P. Selective stabilization of muscle innervation during development: a mathematical model. Biol Cybern. 1983;46(3):207–215. doi: 10.1007/BF00336802. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Apatoff B. R., Heinrich S. P. Suppression of terminal axonal sprouting at the neuromuscular junction by monoclonal antibodies against a muscle-derived antigen of 56,000 daltons. J Cell Biol. 1986 Jun;102(6):2264–2272. doi: 10.1083/jcb.102.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M. E., Heinrich S. P., Lee M. R., Yin H. S. Molecular cloning and expression of neuroleukin, a neurotrophic factor for spinal and sensory neurons. Science. 1986 Oct 31;234(4776):566–574. doi: 10.1126/science.3764429. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Huchet M., Changeux J. P. Neurite-promoting activities for embryonic spinal neurons and their developmental changes in the chick. Dev Biol. 1984 Aug;104(2):336–347. doi: 10.1016/0012-1606(84)90089-7. [DOI] [PubMed] [Google Scholar]
- Jackson P. C. Reduced activity during development delays the normal rearrangement of synapses in the rabbit ciliary ganglion. J Physiol. 1983 Dec;345:319–327. doi: 10.1113/jphysiol.1983.sp014980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. The non-selective innervation of muscle fibres and mixed composition of motor units in a muscle of neonatal rat. J Physiol. 1987 May;386:377–394. doi: 10.1113/jphysiol.1987.sp016539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer J. P., Raper J. A. Collapse of growth cone structure on contact with specific neurites in culture. J Neurosci. 1987 Jan;7(1):201–212. doi: 10.1523/JNEUROSCI.07-01-00201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Magrassi L., Purves D. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci. 1987 Apr;7(4):1215–1222. doi: 10.1523/JNEUROSCI.07-04-01215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S. Properties of motor units in the transversus abdominis muscle of the garter snake. J Physiol. 1987 Dec;393:355–374. doi: 10.1113/jphysiol.1987.sp016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi F. J., Lasek R. J. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987 Aug 7;237(4815):642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbova G. Protease inhibitors reduce the loss of nerve terminals induced by activity and calcium in developing rat soleus muscles in vitro. Neuroscience. 1984 Jun;12(2):637–646. doi: 10.1016/0306-4522(84)90079-4. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester R. R. Competitive elimination of neuromuscular synapses. Nature. 1988 Jan 7;331(6151):21–22. doi: 10.1038/331021a0. [DOI] [PubMed] [Google Scholar]
- Ribchester R. R., Taxt T. Motor unit size and synaptic competition in rat lumbrical muscles reinnervated by active and inactive motor axons. J Physiol. 1983 Nov;344:89–111. doi: 10.1113/jphysiol.1983.sp014926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester R. R., Taxt T. Repression of inactive motor nerve terminals in partially denervated rat muscle after regeneration of active motor axons. J Physiol. 1984 Feb;347:497–511. doi: 10.1113/jphysiol.1984.sp015078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M., Betz W. J. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. J Neurosci. 1984 Oct;4(10):2614–2620. doi: 10.1523/JNEUROSCI.04-10-02614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987 Jul 17;237(4812):291–293. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Taxt T. Local and systemic effects of tetrodotoxin on the formation and elimination of synapses in reinnervated adult rat muscle. J Physiol. 1983 Jul;340:175–194. doi: 10.1113/jphysiol.1983.sp014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Sutton L. A., Riley D. A. Fibre type composition of single motor units during synapse elimination in neonatal rat soleus muscle. Nature. 1984 Jun 21;309(5970):709–711. doi: 10.1038/309709a0. [DOI] [PubMed] [Google Scholar]
- Thompson W. Reinnervation of partially denervated rat soleus muscle. Acta Physiol Scand. 1978 May;103(1):81–91. doi: 10.1111/j.1748-1716.1978.tb06193.x. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Krisch B., Otten U., Thoenen H. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982 Oct 14;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Williams I. R., Gilliatt R. W. Regeneration distal to a prolonged conduction block. J Neurol Sci. 1977 Aug;33(1-2):267–273. doi: 10.1016/0022-510x(77)90199-x. [DOI] [PubMed] [Google Scholar]