Abstract
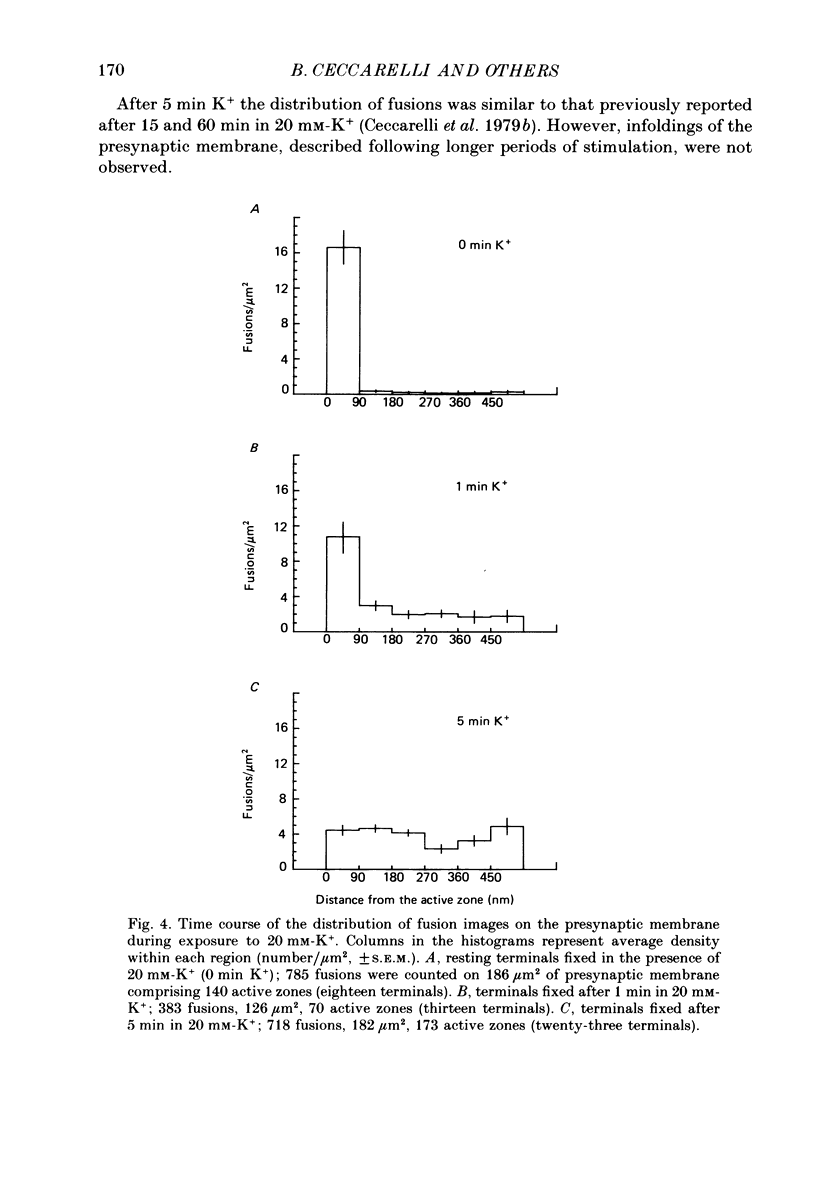
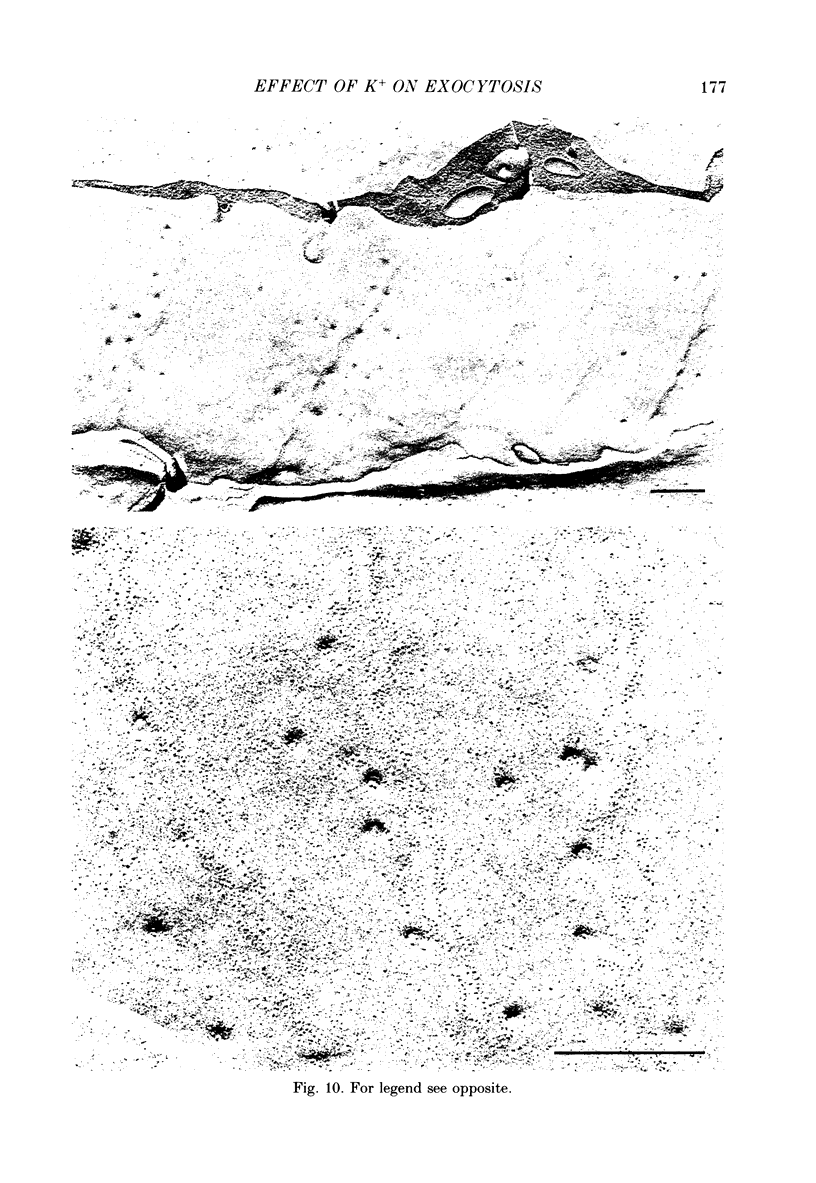
1. Electrophysiology and morphology have been combined to investigate the time course of the exocytosis of quanta of neurotransmitter induced by elevated concentrations of K+ at the frog neuromuscular junction. 2. Replicas of freeze-fractured resting nerve terminals fixed in the presence of 20 mM-K+ showed images of fusion of synaptic vesicles with the presynaptic axolemma which were closely associated with the active zones. After 1 min in 20 nM-K+ fusions appeared also outside the active zones, and by 5 min they became uniformly distributed over the presynaptic membrane. 3. The average total density of fusions was not significantly different at the various times examined since it decreased at the active zones while it increased over the rest of the membrane. 4. Resting terminals fixed in 20 mM-K+ released 33,000-45,000 quanta after the addition of fixative; terminals stimulated by 20 mM-K+ for 1-5 min released 50,000-100,000 quanta during fixation. The fixative potentiated K+-induced transmitter release. 5. Fusions were uniformly distributed in terminals pre-incubated for 5 min in 20 mM-K+ without added Ca2+, stimulated by adding Ca2+ for 30 s, and then fixed. Conversely, after 5 min stimulation in hypertonic Ringer solution fusions remained predominantly located near the active zones. A similar distribution was observed after 15 min stimulation by a lower concentration of K+ (15 mM). 6. At all concentrations of K+ tested (10, 15, 20, 25 mM) miniature end-plate potential (MEPP) rate attained a steady-state value within 10-15 min. Values from a single junction were generally lower at higher concentrations of K+, which indicates partial inactivation of the secretion-recycling process. 7. The data indicate that K+ initially activates exocytosis at the active zones. Subsequently, ectopic exocytosis is activated while sites at the active zones appear to undergo partial inactivation. These phenomena are not related to the intensity or to the amount of previous secretion.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. I. Effects of black widow spider venom and Ca2+-free solutions on the structure of the active zone. J Cell Biol. 1979 Apr;81(1):163–177. doi: 10.1083/jcb.81.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. II. Effects of electrical stimulation and high potassium. J Cell Biol. 1979 Apr;81(1):178–192. doi: 10.1083/jcb.81.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Clark A. W. Changes in the structure of neuromuscular junctions caused by variations in osmotic pressure. J Cell Biol. 1976 Jun;69(3):521–538. doi: 10.1083/jcb.69.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. The specific effect of potassium on transmitter release by motor nerve terminals and its inhibition by calcium. J Physiol. 1973 Jan;228(2):435–458. doi: 10.1113/jphysiol.1973.sp010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R., Segal J. R., Ceccarelli B., Hurlbut W. P. Effects of black widow spider venom and Ca2+ on quantal secretion at the frog neuromuscular junction. J Gen Physiol. 1986 Jul;88(1):59–81. doi: 10.1085/jgp.88.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R., Segal J. R., Hurlbut W. P. Fluctuation analysis of nonideal shot noise. Application to the neuromuscular junction. J Gen Physiol. 1986 Jul;88(1):25–57. doi: 10.1085/jgp.88.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Dual effect of potassium on transmitter release. Nature. 1965 May 8;206(984):625–626. doi: 10.1038/206625a0. [DOI] [PubMed] [Google Scholar]
- Haimann C., Torri-Tarelli F., Fesce R., Ceccarelli B. Measurement of quantal secretion induced by ouabain and its correlation with depletion of synaptic vesicles. J Cell Biol. 1985 Nov;101(5 Pt 1):1953–1965. doi: 10.1083/jcb.101.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Jehl B., Bauer R., Dörge A., Rick R. The use of propane/isopentane mixtures for rapid freezing of biological specimens. J Microsc. 1981 Sep;123(Pt 3):307–309. doi: 10.1111/j.1365-2818.1981.tb02475.x. [DOI] [PubMed] [Google Scholar]
- Kita H., van der Kloot W. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. J Neurophysiol. 1977 Mar;40(2):212–224. doi: 10.1152/jn.1977.40.2.212. [DOI] [PubMed] [Google Scholar]
- Miller T. M., Heuser J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984 Feb;98(2):685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J. R., Ceccarelli B., Fesce R., Hurlbut W. P. Miniature endplate potential frequency and amplitude determined by an extension of Campbell's theorem. Biophys J. 1985 Feb;47(2 Pt 1):183–202. doi: 10.1016/s0006-3495(85)83891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Reese T. S. Use of aldehyde fixatives to determine the rate of synaptic transmitter release. J Exp Biol. 1980 Dec;89:19–29. doi: 10.1242/jeb.89.1.19. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri-Tarelli F., Grohovaz F., Fesce R., Ceccarelli B. Temporal coincidence between synaptic vesicle fusion and quantal secretion of acetylcholine. J Cell Biol. 1985 Oct;101(4):1386–1399. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri-Tarelli F., Haimann C., Ceccarelli B. Coated vesicles and pits during enhanced quantal release of acetylcholine at the neuromuscular junction. J Neurocytol. 1987 Apr;16(2):205–214. doi: 10.1007/BF01795304. [DOI] [PubMed] [Google Scholar]
- Valtorta F., Madeddu L., Meldolesi J., Ceccarelli B. Specific localization of the alpha-latrotoxin receptor in the nerve terminal plasma membrane. J Cell Biol. 1984 Jul;99(1 Pt 1):124–132. doi: 10.1083/jcb.99.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]







